54868-4983 : Oxycodone Hydrochloride 5 mg Oral Tablet
| NDC: | 54868-4983 |
| Labeler: | Physicians Total Care, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Oxycodone Hydrochloride Oxycodone Hydrochloride |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA077712 |
| Rev. Date: | |
| CSA Schedule: | CII (US) [1] |
[1] Schedule II / IIN Controlled Substance: High potential for abuse which may lead to severe psychological or physical dependence. (i.e. Narcotics such as Dilaudid, Methadone, Demerol, Oxycodone, Percocet, Fentanyl, Morphine, Opium, Codeine, and Hydrocodone ... Schedule IIN stimulants include non-narcotic Amphetamines such as Dexedrine, Adderall, Desoxyn, Methylphenidate (Ritalin) ... Other Schedule II substances include Amobarbital, Glutethimide, and Pentobarbital. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | 4810;V |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 6 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
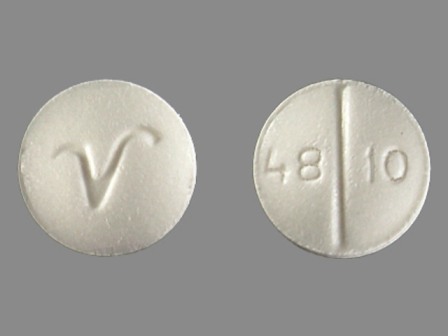
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54868-4983-0: 60 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑0)
- 54868-4983-1: 50 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑1)
- 54868-4983-2: 20 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑2)
- 54868-4983-3: 90 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑3)
- 54868-4983-4: 30 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑4)
- 54868-4983-5: 100 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑5)
- 54868-4983-8: 120 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑8)
- 54868-4983-9: 150 TABLET IN 1 BOTTLE, PLASTIC (54868‑4983‑9)
Active Ingredients:
- Oxycodone Hydrochloride
Dosage Strength:
- 5 mg
Inactive Ingredients:
- Lactose Monohydrate
- Magnesium Stearate
- Cellulose, Microcrystalline
- Povidone
- Sodium Starch Glycolate Type a Potato
- Stearic Acid
Pharmaceutical Classes:
- Full Opioid Agonists [MoA]
- Opioid Agonist [EPC]
Related Products:
Based on records with the same trade name.- 54868-3137 Oxycodone Hydrochloride 10 mg Oral Tablet by Physicians Total Care, Inc.
- 54868-4980 Oxycodone Hydrochloride 15 mg Oral Tablet by Physicians Total Care, Inc.
- 54868-5390 Oxycodone Hydrochloride 30 mg Oral Tablet by Physicians Total Care, Inc.
- 54868-5902 Oxycodone Hydrochloride 20 mg Oral Tablet by Physicians Total Care, Inc.
- 0054-0390 Oxycodone Hydrochloride 5 mg/5ml Oral Solution by Roxane Laboratories, Inc.
- 0054-0393 Oxycodone Hydrochloride 100 mg/5ml Oral Solution by Roxane Laboratories, Inc.
- 0054-0522 Oxycodone Hydrochloride 100 mg/5ml Oral Solution by Roxane Laboratories, Inc.
- 0054-0523 Oxycodone Hydrochloride 5 mg/5ml Oral Solution by Roxane Laboratories, Inc.
- 0093-5731 Oxycodone Hydrochloride 10 mg Oral Tablet, Film Coated, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5732 Oxycodone Hydrochloride 20 mg Oral Tablet, Film Coated, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5733 Oxycodone Hydrochloride 40 mg Oral Tablet, Film Coated, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5734 Oxycodone Hydrochloride 80 mg Oral Tablet, Film Coated, Extended Release by Teva Pharmaceuticals USA Inc
- 0115-1556 Oxycodone Hydrochloride 10 mg Oral Tablet, Film Coated, Extended Release by Impax Generics
- 0115-1557 Oxycodone Hydrochloride 15 mg Oral Tablet, Film Coated, Extended Release by Impax Generics
- 0115-1558 Oxycodone Hydrochloride 20 mg Oral Tablet, Film Coated, Extended Release by Impax Generics
- 0115-1559 Oxycodone Hydrochloride 30 mg Oral Tablet, Film Coated, Extended Release by Impax Generics
- 0115-1560 Oxycodone Hydrochloride 40 mg Oral Tablet, Film Coated, Extended Release by Impax Generics
- 0115-1561 Oxycodone Hydrochloride 60 mg Oral Tablet, Film Coated, Extended Release by Impax Generics
- 0115-1562 Oxycodone Hydrochloride 80 mg Oral Tablet, Film Coated, Extended Release by Impax Generics
- 0121-0826 Oxycodone Hydrochloride 100 mg/5ml Oral Solution by Pharmaceutical Associates, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54868-4981Next: 54868-4984 >
Related Discussions:
Oxycodone 30mg pain clinics
i have been having trouble getting a good dr who will write oxycodone 30 mg. my back has been broken before and i was go... 1631 replies
i have been having trouble getting a good dr who will write oxycodone 30 mg. my back has been broken before and i was go... 1631 replies
Oxycodone Doctor in NJ
I need a doctor that will prescribe oxycodone 30mg in New Jersey. My doctor recently took me off of 30mg oxycodone becau... 249 replies
I need a doctor that will prescribe oxycodone 30mg in New Jersey. My doctor recently took me off of 30mg oxycodone becau... 249 replies
Oxycodone blue pill a 51
How effective are the A51 blue oxycodone pills? Does it work like the mbox 30's V's Or A215??? ## Hello, Chris! ... 145 replies
How effective are the A51 blue oxycodone pills? Does it work like the mbox 30's V's Or A215??? ## Hello, Chris! ... 145 replies
Oxycodone/Percocet generics! Which are most effective?
Hello, First, let me start by saying that I am somewhat opiate naive. I am not in pain management and hopefully won'... 143 replies
Hello, First, let me start by saying that I am somewhat opiate naive. I am not in pain management and hopefully won'... 143 replies
Oxycodone Vs Fentanyl Withdrawals
I have just switched from oxycodone to fentanyl patches. I will be withdrawing from them following sugery. any suggestio... 74 replies
I have just switched from oxycodone to fentanyl patches. I will be withdrawing from them following sugery. any suggestio... 74 replies
Oxycodone Doctors in Louisiana
I've got an mri reporty, and the mri itself on a cd, and live in southern louisiana. a lot of ppl used to go to hous... 74 replies
I've got an mri reporty, and the mri itself on a cd, and live in southern louisiana. a lot of ppl used to go to hous... 74 replies
Oxycodone's Deathly Issue
I've been using Oxycodone for five years for pain management. I read about all the deaths that were associated with ... 69 replies
I've been using Oxycodone for five years for pain management. I read about all the deaths that were associated with ... 69 replies
Oxycodone Shortage In Florida 2012
Can a pharmacist just be quite and fill the script the doctor ordered for his patient.They lie to us and then fill freds... 64 replies
Can a pharmacist just be quite and fill the script the doctor ordered for his patient.They lie to us and then fill freds... 64 replies
oxycodone 20 mg K57 vs NP14
I don't usually count the pills when I pick up my Rx. This time I did & felt a difference in the thickness. Some... 62 replies
I don't usually count the pills when I pick up my Rx. This time I did & felt a difference in the thickness. Some... 62 replies
oxycodone blue pill a 51 fake?
So confused.. There are some of these that are really crumbly and look a lot darker than the ones I originally saw. They... 60 replies
So confused.. There are some of these that are really crumbly and look a lot darker than the ones I originally saw. They... 60 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.