68151-0528 : Disopyramide Phosphate 150 mg Oral Capsule
| NDC: | 68151-0528 |
| Labeler: | Carilion Materials Management |
| Product Type: | Human Prescription Drug |
| Drug Name: | Disopyramide Phosphate |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA070102 |
| Rev. Date: |
Appearance:
| Markings: | 93;3129 |
| Shapes: |
Capsule |
| Colors: |
 Brown / Brown /
 Red Red |
| Size (mm): | 19 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
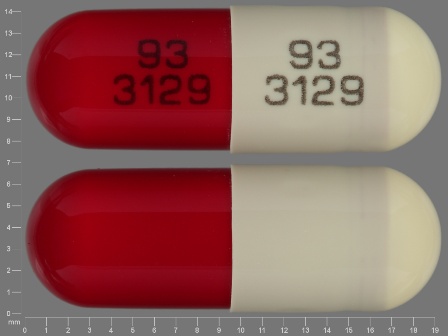
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 68151-0528-0: 1 CAPSULE IN 1 PACKAGE (68151‑0528‑0)
Active Ingredients:
- Disopyramide Phosphate
Dosage Strength:
- 150 mg
Inactive Ingredients:
- Lactose Monohydrate
- Magnesium Stearate
- Sodium Starch Glycolate Type a Potato
- Ferrosoferric Oxide
- D&c Red No. 28
- D&c Red No. 33
- D&c Yellow No. 10
- Aluminum Oxide
- Fd&c Blue No. 1
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Gelatin
- Propylene Glycol
- Shellac
- Sodium Lauryl Sulfate
- Sorbitan Monolaurate
- Titanium Dioxide
Pharmaceutical Classes:
- Antiarrhythmic [EPC]
Related Products:
Based on records with the same trade name.- 68151-3858 Disopyramide Phosphate 100 mg Oral Capsule by Carilion Materials Management
- 0093-3127 Disopyramide 100 mg (As Disopyramide Phosphate) Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-3129 Disopyramide 150 mg (As Disopyramide Phosphate) Oral Capsule by Teva Pharmaceuticals USA Inc
- 0591-5560 Disopyramide 100 mg (As Disopyramide Phosphate) Oral Capsule by Watson Laboratories, Inc.
- 0591-5561 Disopyramide 150 mg (As Disopyramide Phosphate) Oral Capsule by Watson Laboratories, Inc.
- 42291-234 Disopyramide Phosphate 100 mg/1 Oral Capsule by Avkare, Inc.
- 42291-235 Disopyramide Phosphate 150 mg/1 Oral Capsule by Avkare, Inc.
- 51407-131 Disopyramide Phosphate 100 mg Oral Capsule by Golden State Medical Supply Inc.
- 51407-132 Disopyramide Phosphate 150 mg Oral Capsule by Golden State Medical Supply Inc.
- 51862-093 Disopyramide Phosphate 100 mg Oral Capsule by Mayne Pharma Inc.
- 51862-094 Disopyramide Phosphate 150 mg Oral Capsule by Mayne Pharma Inc.
- 51862-095 Disopyramide Phosphate 100 mg Oral Capsule by Mayne Pharma Inc.
- 51862-096 Disopyramide Phosphate 150 mg Oral Capsule by Mayne Pharma Inc.
- 59762-0386 Disopyramide Phosphate 100 mg Oral Capsule, Gelatin Coated by Greenstone LLC
- 59762-0400 Disopyramide Phosphate 150 mg Oral Capsule, Gelatin Coated by Greenstone LLC
- 60429-984 Disopyramide Phosphate 100 mg Oral Capsule by Gsms, Incorporated
- 60429-985 Disopyramide Phosphate 150 mg Oral Capsule by Gsms, Incorporated
- 75907-026 Disopyramide Phosphate 150 mg Oral Capsule by Dr. Reddy's Labratories Inc.
- 75907-027 Disopyramide Phosphate 100 mg Oral Capsule by Dr. Reddy's Labratories Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 68151-0527Next: 68151-0537 >
Related Discussions:
codeine phosphate
little white tablrt t ## Can anyone tell me if Codeine Phosphate 30 mg tablets will relieve the pain that a percocet 10/... 4 replies
little white tablrt t ## Can anyone tell me if Codeine Phosphate 30 mg tablets will relieve the pain that a percocet 10/... 4 replies
Codeine Phosphate 30g
can you tell me how many of these tablets would result in an overdose? ## There is really no definitive answer to such a... 3 replies
can you tell me how many of these tablets would result in an overdose? ## There is really no definitive answer to such a... 3 replies
codeine phosphate clorphenramine syrup
I am taking codeine syrup taking 100 ml in a day. What are its side effects and how should I go about the withdrawal pro... 1 reply
I am taking codeine syrup taking 100 ml in a day. What are its side effects and how should I go about the withdrawal pro... 1 reply
codiene phosphate dpi
red and white capsule imprint dpi 364 ## Based on the description provided, I found your pill to be Duradrin; which is a... 1 reply
red and white capsule imprint dpi 364 ## Based on the description provided, I found your pill to be Duradrin; which is a... 1 reply
Codeine Phosphate Ta 30mg Cod
is it ok to drink alcohol whilst taking codeine phosphate 30mg 4 times a day ## It is not recommended that you use alcoh... 1 reply
is it ok to drink alcohol whilst taking codeine phosphate 30mg 4 times a day ## It is not recommended that you use alcoh... 1 reply
chloroquine phosphate
Arthritis ## Chloroquine phosphate has long been used in the treatment or prevention of malaria. As it mildly suppresses... 7 replies
Arthritis ## Chloroquine phosphate has long been used in the treatment or prevention of malaria. As it mildly suppresses... 7 replies
sitagliptin phosphate metformin hydrochloride tablet
Sitagliptin phosphate mrtoformin hydrochloride istamet tablet should be takeb before or after food ## Unless your doctor... 1 reply
Sitagliptin phosphate mrtoformin hydrochloride istamet tablet should be takeb before or after food ## Unless your doctor... 1 reply
clindamycin phosphate gel expiration date
Hello, I have a 1 year old clindamycin phosphate gel tube that I am considering using. My acne is acting up and I would ... 1 reply
Hello, I have a 1 year old clindamycin phosphate gel tube that I am considering using. My acne is acting up and I would ... 1 reply
Is clindamycin phosphate lotion1% safe to use on a 13 lb dog
My bichon has red on the inside of his ears and a hot spot below his ear another hot spot on his neck. I put this lotion... 3 replies
My bichon has red on the inside of his ears and a hot spot below his ear another hot spot on his neck. I put this lotion... 3 replies
taking codeine phosphate to help come off subutex
Hi, I was prescribed 180mg of codeine phosphate for on-going back pain, BUT I decided to give subutex a go, as advised b... 1 reply
Hi, I was prescribed 180mg of codeine phosphate for on-going back pain, BUT I decided to give subutex a go, as advised b... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
