Viramune
Active Ingredient(s): NevirapineFDA Approved: * June 21, 1996
Pharm Company: * BOEHRINGER INGELHEIM
Category: HIV / AIDS
Nevirapine (NVP), sold under the brand name Viramune among others, is a medication used to treat and prevent HIV/AIDS, specifically HIV-1.[3] It is generally recommended for use with other antiretroviral medications.[3] It may be used to prevent mother to child spread during birth but is not recommended following other exposures.[3] It is taken by mouth.[3] Common side effects include rash, headache, nausea, feeling tired, and li... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List
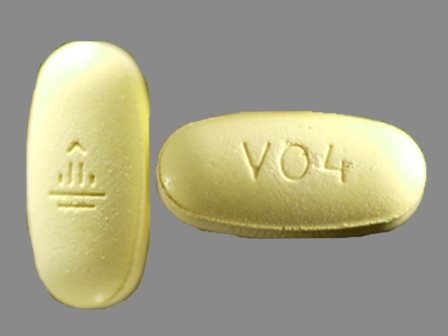
24 Hr Viramune 400 mg Extended Release Tablet
NDC: 0597-0123
Labeler:
Boehringer Ingelheim Pharmaceuticals, Inc.




Viramune 50 mg/5ml Oral Suspension
NDC: 0597-0047
Labeler:
Boehringer Ingelheim Pharmaceuticals Inc.
24 Hr Viramune 100 mg Extended Release Tablet
NDC: 0597-0129
Labeler:
Boehringer Ingelheim Pharmaceuticals, Inc.