53808-0808 : Viramune 200 mg Oral Tablet
| NDC: | 53808-0808 |
| Labeler: | State of Florida Doh Central Pharmacy |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Viramune Viramune |
| Dosage Form: | Oral Tablet |
| Application #: | NDA020636 |
| Rev. Date: |
Appearance:
| Markings: | 54;193 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 19 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
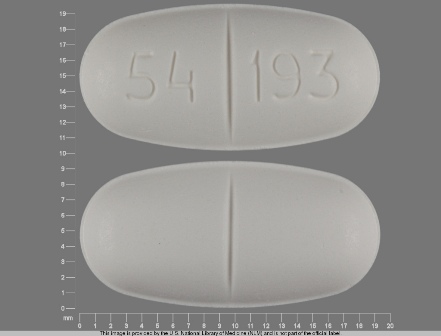
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 53808-0808-1: 30 TABLET IN 1 BLISTER PACK (53808‑0808‑1)
Active Ingredients:
- Nevirapine
Dosage Strength:
- 200 mg
Inactive Ingredients:
- Cellulose, Microcrystalline
- Lactose Monohydrate
- Povidone
- Sodium Starch Glycolate Type a Potato
- Silicon Dioxide
- Magnesium Stearate
Pharmaceutical Classes:
- Human Immunodeficiency Virus 1 Non-Nucleoside Analog Reverse Transcriptase Inhibitor [EPC]
- Non-Nucleoside Analog [EXT]
- Non-Nucleoside Reverse Transcriptase Inhibitors [MoA]
- Cytochrome P450 3A Inducers [MoA]
- Cytochrome P450 2B6 Inducers [MoA]
Related Products:
Based on records with the same trade name.- 0597-0046 Viramune 200 mg Oral Tablet by Boehringer Ingelheim Pharmaceuticals Inc.
- 0597-0047 Viramune 50 mg/5ml Oral Suspension by Boehringer Ingelheim Pharmaceuticals Inc.
- 0597-0123 24 Hr Viramune 400 mg Extended Release Tablet by Boehringer Ingelheim Pharmaceuticals, Inc.
- 0597-0129 24 Hr Viramune 100 mg Extended Release Tablet by Boehringer Ingelheim Pharmaceuticals, Inc.
- 54569-4561 Viramune 200 mg Oral Tablet by A-s Medication Solutions
- 54569-6236 Viramune 400 mg Oral Tablet, Extended Release by A-s Medication Solutions
- 54868-6370 24 Hr Viramune 400 mg Extended Release Tablet by Physicians Total Care, Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 53808-0807Next: 53808-0809 >
Related Discussions:
ILVITRIMl / Nevirapine
Hi after six weeks checkup my daughter tested negative, before she was using Nevirapine. Now they gave her the ilvitrim ... 3 replies
Hi after six weeks checkup my daughter tested negative, before she was using Nevirapine. Now they gave her the ilvitrim ... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.