Tacrolimus
FDA Approved: * August 10, 2009Pharm Company: * SANDOZ
Category: Skin Care
Tacrolimus, sold under the brand name Prograf among others, is an immunosuppressive drug. After allogeneic organ transplant, the risk of organ rejection is moderate. To lower the risk of organ rejection, tacrolimus is given. The drug can also be sold as a topical medication in the treatment of T-cell-mediated diseases such as eczema and psoriasis. For example, it is prescribed for severe refractory uveitis after a bone marrow transplant, exacerbations of minimal change disease, Kimura's disea... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.10 Discussions
Dosage List
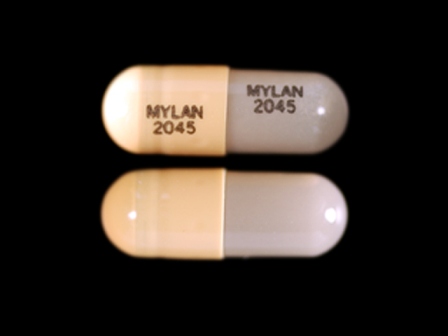


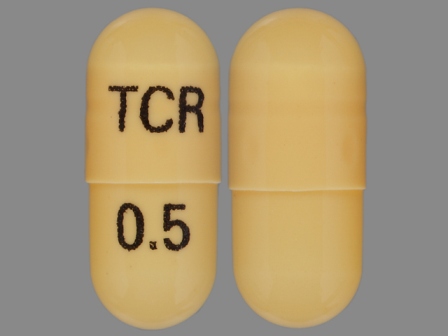
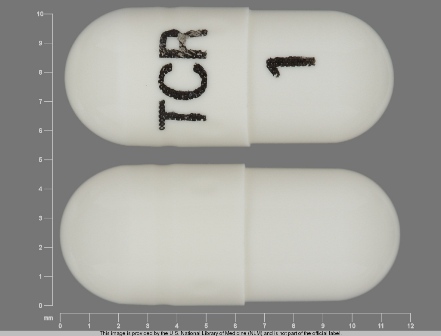
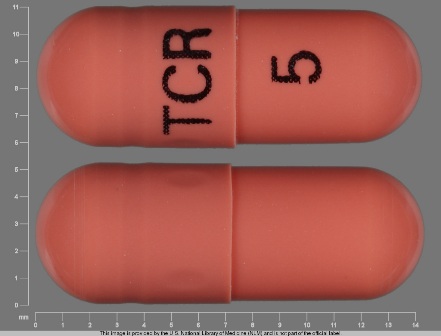
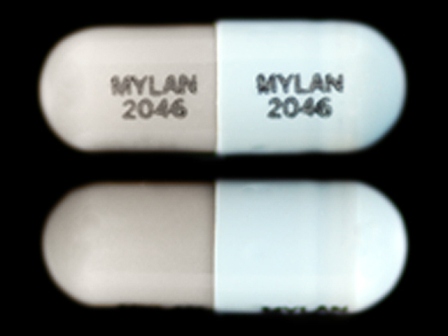
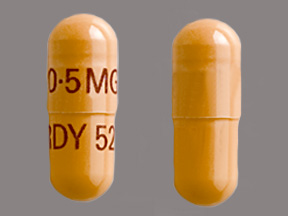
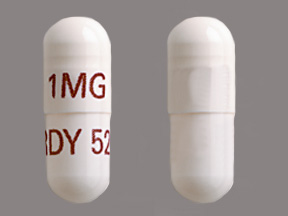

Related Brands
Drugs with the same active ingredientsPopular Topics
Are there any withdrawals or side effects from stopping Tacrolimus? I had a heart tx and I'm still on Rappamune, Cel...
Can I take a tonic that includes pomegranate supplement when taking Tacrolimus and Mycophenolate anti-kidney-rejection m...
I had a kidney transplant and am on the following medications: Tacrolimus, cellcept, amlodipine, atenolol, bactrim &...
I am a good friend and caregiver for a man who was given the new gift of life. The down side is the side effects of this...
Tacrolimus Ointment 0 1% w/w is help full for depigmentation of skin..??...
please send the tacrolimus assay method for research work...
Does anyone know how to remove the excipients from tacrolimus prior to running it on HPLC? I have found that susequent r...
kidney transplant sapresant...