Prograf
Active Ingredient(s): TacrolimusFDA Approved: * April 8, 1994
Pharm Company: * ASTELLAS
Category: Skin Care
Tacrolimus, sold under the brand name Prograf among others, is an immunosuppressive drug. After allogeneic organ transplant, the risk of organ rejection is moderate. To lower the risk of organ rejection, tacrolimus is given. The drug can also be sold as a topical medication in the treatment of T-cell-mediated diseases such as eczema and psoriasis. For example, it is prescribed for severe refractory uveitis after a bone marrow transplant, exacerbations of minimal change disease, Kimura's disea... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.6 Discussions
Dosage List
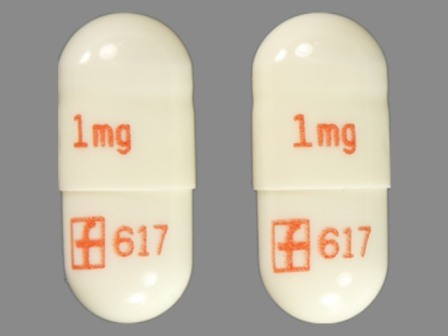

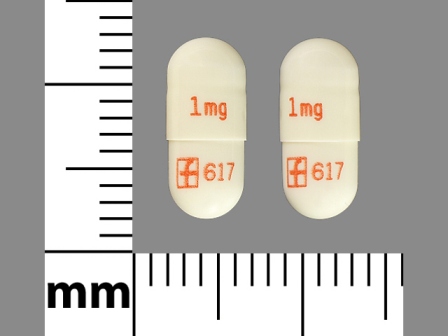
Prograf 1 mg Oral Capsule, Gelatin Coated
NDC: 43353-178
Labeler:
Aphena Pharma Solutions - Tennessee, LLC


Related Brands
Drugs with the same active ingredientsPopular Topics
About Side Effect Of Taking Sensa When Prograf 1 REPLY
1 REPLY
I had a kidney transplant aug 2011, I gain a lot of weight and thinking of taking sensa to help me loose weigh. Currentl...
prograf
white capsule...
cell cept prograf
anti rejection...