Entecavir
FDA Approved: * August 26, 2014Pharm Company: * TEVA PHARMS USA
Category: Hepatitis
Entecavir (ETV), sold under the brand name Baraclude, is an antiviral medication used in the treatment of hepatitis B virus (HBV) infection.[4] In those with both HIV/AIDS and HBV antiretroviral medication should also be used.[4] Entecavir is taken by mouth as a tablet or solution.[4] Common side effects include headache, nausea, high blood sugar, and decreased kidney function.[4] Severe side effects include enlargement of the li... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List
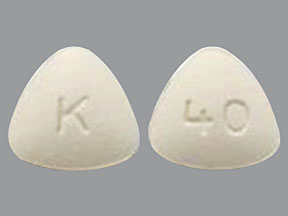


Related Brands
Drugs with the same active ingredientsPopular Topics
LN beta 2.5 mg and Baraclude (Entecavir) 1 REPLY
1 REPLY
My mother (age 50) has hepatitis B. She is on Baraclude (Entecavir). Recently she was diagnosed with high blood pressure...