49884-105 : Entecavir 1 mg Oral Tablet, Film Coated
| NDC: | 49884-105 |
| Labeler: | Par Pharmaceutical, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Entecavir Entecavir |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA021797 |
| Rev. Date: |
Appearance:
| Markings: | BMS;1612 |
| Shapes: |
Triangle (3 sides) |
| Colors: |
 Pink Pink |
| Size (mm): | 10 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
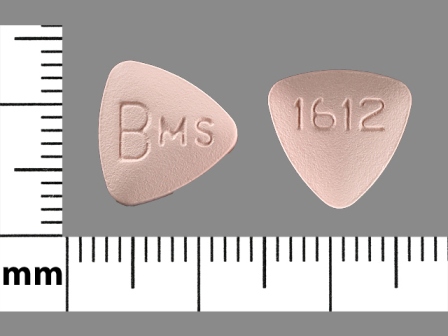
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 49884-105-11: 1 BOTTLE IN 1 CARTON (49884‑105‑11) > 30 TABLET, FILM COATED IN 1 BOTTLE
Active Ingredients:
- Entecavir
Dosage Strength:
- 1 mg
Inactive Ingredients:
- Lactose Monohydrate
- Cellulose, Microcrystalline
- Crospovidone
- Povidones
- Magnesium Stearate
- Titanium Dioxide
- Hypromelloses
- Polyethylene Glycol 400
- Ferric Oxide Red
Pharmaceutical Classes:
- Hepatitis B Virus Nucleoside Analog Reverse Transcriptase Inhibitor [EPC]
- Nucleoside Analog [Chemical/Ingredient]
- Nucleoside Reverse Transcriptase Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 49884-104 Entecavir .5 mg Oral Tablet, Film Coated by Par Pharmaceutical, Inc.
- 49884-547 Entecavir .5 mg Oral Tablet, Coated by Par Pharmaceutical Inc.
- 49884-548 Entecavir 1 mg Oral Tablet, Coated by Par Pharmaceutical Inc.
- 0093-5786 Entecavir .5 mg Oral Tablet, Film Coated by Teva Pharmaceuticals USA Inc
- 0093-5787 Entecavir 1 mg Oral Tablet, Film Coated by Teva Pharmaceuticals USA Inc
- 10135-615 Entecavir .5 mg Oral Tablet, Film Coated by Marlex Pharmaceuticals Inc
- 10135-616 Entecavir 1 mg Oral Tablet, Film Coated by Marlex Pharmaceuticals Inc
- 16714-717 Entecavir .5 mg Oral Tablet, Film Coated by Northstar Rxllc
- 16714-718 Entecavir 1 mg Oral Tablet, Film Coated by Northstar Rxllc
- 16729-388 Entecavir .5 mg Oral Tablet, Film Coated by Accord Healthcare Inc.
- 16729-389 Entecavir 1 mg Oral Tablet, Film Coated by Accord Healthcare Inc.
- 31722-833 Entecavir .5 mg Oral Tablet by Camber Pharmaceuticals, Inc.
- 31722-834 Entecavir 1 mg Oral Tablet by Camber Pharmaceuticals, Inc.
- 42291-261 Entecavir .5 mg Oral Tablet, Film Coated by Avkare, Inc.
- 42291-262 Entecavir 1 mg Oral Tablet, Film Coated by Avkare, Inc.
- 42494-457 Entecavir .5 mg Oral Tablet by Cameron Pharmaceuticals
- 42494-458 Entecavir 1 mg Oral Tablet by Cameron Pharmaceuticals
- 42806-658 Entecavir .5 mg Oral Tablet by Epic Pharma, LLC
- 42806-659 Entecavir 1 mg Oral Tablet by Epic Pharma, LLC
- 43547-436 Entecavir .5 mg Oral Tablet, Film Coated by Solco Healthcare LLC
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 49884-104Next: 49884-106 >
Related Discussions:
LN beta 2.5 mg and Baraclude (Entecavir)
My mother (age 50) has hepatitis B. She is on Baraclude (Entecavir). Recently she was diagnosed with high blood pressure... 1 reply
My mother (age 50) has hepatitis B. She is on Baraclude (Entecavir). Recently she was diagnosed with high blood pressure... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.