Complera
Active Ingredient(s): Emtricitabine + Rilpivirine + Tenofovir Disoproxil FumarateFDA Approved: * August 10, 2011
Pharm Company: * GILEAD
Category: HIV / AIDS
Emtricitabine/rilpivirine/tenofovir (trade names Complera, Eviplera) is a fixed-dose combination of antiretroviral drugs for the treatment of HIV/AIDS.[1] The drug was co-developed by Gilead Sciences and Johnson & Johnson's Tibotec division and was approved by the Food and Drug Administration in August 2011, and by the European Medicines Agency in November 2011,[2] for patients who have not previously been treated for HIV.[3] It is availab... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List
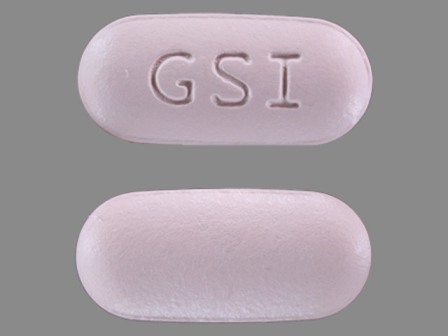
Complera (Emtricitabine 200 mg / Rilpivirine 25 mg / Tenofovir Disoproxil Fumarate 300 mg) Oral Tablet
NDC: 61958-1101
Labeler:
Gilead Sciences, Inc.



Complera (Emtricitabine 200 mg / Rilpivirine 25 mg / Tenofovir Disoproxil Fumarate 300 mg) Oral Tablet
NDC: 54868-6360
Labeler:
Physicians Total Care, Inc.

Popular Topics
up john Complera / 5HTP 6 REPLIES
6 REPLIES
I've been doing very well with Complera since January. I have been wondering if I can take 5HTP with it. I am a 50 y...