50090-1248 : Complera Oral Tablet, Film Coated
| NDC: | 50090-1248 |
| Labeler: | A-s Medication Solutions |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Complera Complera |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA202123 |
| Rev. Date: |
Appearance:
| Markings: | GSI |
| Shapes: |
Oval |
| Colors: |
 Purple Purple |
| Size (mm): | 19 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
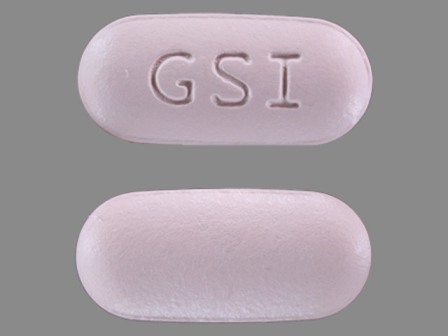
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50090-1248-0: 30 TABLET, FILM COATED IN 1 BOTTLE (50090‑1248‑0)
Active Ingredients:
- Emtricitabine
- Rilpivirine Hydrochloride
- Tenofovir Disoproxil Fumarate
Dosage Strength:
- 200 mg
- 25 mg
- 300 mg
Inactive Ingredients:
- Microcrystalline Cellulose
- Lactose Monohydrate
- Povidone, Unspecified
- Polysorbate 20
- Croscarmellose Sodium
- Magnesium Stearate
- Starch, Corn
- Hypromellose, Unspecified
- Fd&c Blue No. 2
- Aluminum Oxide
- Polyethylene Glycol, Unspecified
- Ferric Oxide Red
- Fd&c Yellow No. 6
- Titanium Dioxide
- Triacetin
Pharmaceutical Classes:
- Human Immunodeficiency Virus Nucleoside Analog Reverse Transcriptase Inhibitor [EPC]
- Nucleoside Analog [Chemical/Ingredient]
- Nucleoside Reverse Transcriptase Inhibitors [MoA]
- Human Immunodeficiency Virus 1 Non-Nucleoside Analog Reverse Transcriptase Inhibitor [EPC]
- Non-Nucleoside Reverse Transcriptase Inhibitors [MoA]
- Non-Nucleoside Analog [Chemical/Ingredient]
- Hepatitis B Virus Nucleoside Analog Reverse Transcriptase Inhibitor [EPC]
Related Products:
Based on records with the same trade name.- 54569-6270 Complera Oral Tablet, Film Coated by A-s Medication Solutions
- 54868-6360 Complera (Emtricitabine 200 mg / Rilpivirine 25 mg / Tenofovir Disoproxil Fumarate 300 mg) Oral Tablet by Physicians Total Care, Inc.
- 61958-1101 Complera (Emtricitabine 200 mg / Rilpivirine 25 mg / Tenofovir Disoproxil Fumarate 300 mg) Oral Tablet by Gilead Sciences, Inc.
- 70518-0677 Complera Oral Tablet, Film Coated by Remedyrepack Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50090-1247Next: 50090-1250 >
Related Discussions:
up john Complera / 5HTP
I've been doing very well with Complera since January. I have been wondering if I can take 5HTP with it. I am a 50 y... 6 replies
I've been doing very well with Complera since January. I have been wondering if I can take 5HTP with it. I am a 50 y... 6 replies
Tenofovir with primolut N
Actually I am taking tenofovir from the past 2 years. Now I need to take primolut N. Can I take it with tenofovir? One o...
Actually I am taking tenofovir from the past 2 years. Now I need to take primolut N. Can I take it with tenofovir? One o...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.