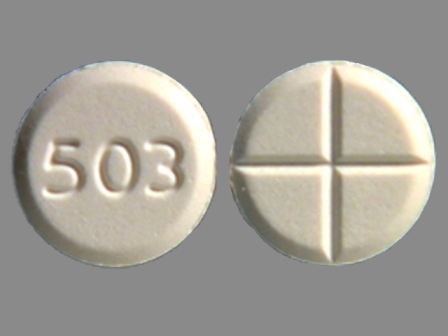
Tizanidine - NDC Database (Page 4)
330 records found
Start a Discussion
63187-673 Dec 11, 2003
Tizanidine 4 mg Oral Tablet by Proficient Rx Lp
63629-3209 Jul 03, 2002
Tizanidine 2 mg (Tizanidine Hydrochloride 2.29 mg) Oral Tablet by Bryant Ranch Prepack
63629-6537 Jun 27, 2002
Tizanidine Hydrochloride 4 mg Oral Tablet by Bryant Ranch Prepack
63874-087 Jan 01, 2010
Tizanidine 2 mg (Tizanidine Hydrochloride 2.29 mg) Oral Tablet by Altura Pharmaceuticals, Inc.
68071-3288 Jan 16, 2004
Tizanidine 2 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
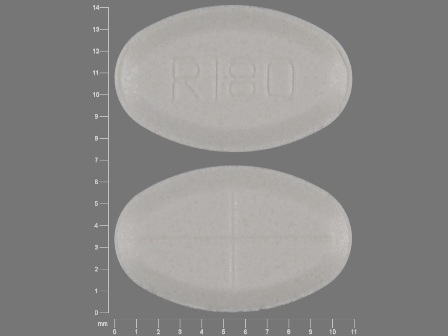
68084-013 Jul 08, 2013
Tizanidine 4 mg (As Tizanidine Hydrochloride 4.58 mg) Oral Tablet by American Health Packaging
68788-6365 Mar 16, 2016
Tizanidine 4 mg Oral Tablet by Preferred Pharmaceuticals Inc.
68788-6431 Jun 28, 2016
Tizanidine Hydrochloride 2 mg Oral Tablet by Preferred Pharmaceuticals Inc.
68788-9091 Jul 03, 2002
Tizanidine 2 mg (Tizanidine Hydrochloride 2.29 mg) Oral Tablet by Preferred Pharmaceuticals, Inc
68788-9497 Mar 26, 2014
Tizanidine 2 mg Oral Tablet by Preferred Pharmaceuticals, Inc.
70518-1439 Sep 27, 2018
Tizanidine 4 mg Oral Tablet by Remedyrepack Inc.
70934-268 Feb 15, 2019
Tizanidine 4 mg Oral Tablet by Denton Pharma, Inc. Dba Northwind Pharmaceuticals
71335-0003 Jan 16, 2004
Tizanidine 2 mg Oral Tablet by Bryant Ranch Prepack
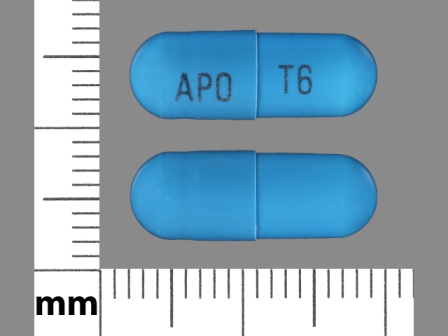
71335-0317 Feb 06, 2012
Tizanidine Hydrochloride 6 mg Oral Capsule, Gelatin Coated by Bryant Ranch Prepack
71335-1016 Dec 11, 2003
Tizanidine 4 mg Oral Tablet by Bryant Ranch Prepack
71610-114 Jun 27, 2002
Tizanidine Hydrochloride 4 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, LLC
71610-167 Jul 28, 2015
Tizanidine 4 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, LLC
71610-451 Dec 11, 2003
Tizanidine 4 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, LLC
71610-455 Jan 16, 2004
Tizanidine Hydrochloride by Aphena Pharma Solutions - Tennessee, LLC
75834-207 Sep 26, 2019
Tizanidine Hydrochloride 2 mg Oral Capsule by Nivagen Pharmaceuticals, Inc.
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .