186 records found
Page: 1 Start a Discussion
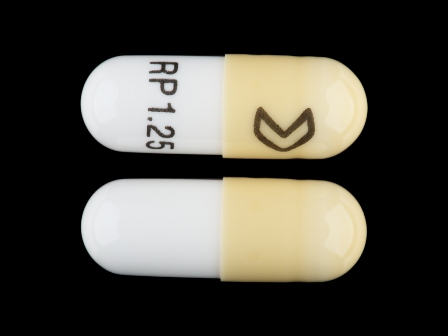
0054-0106 Jun 18, 2008
Ramipril 1.25 mg Oral Capsule by Roxane Laboratories, Inc
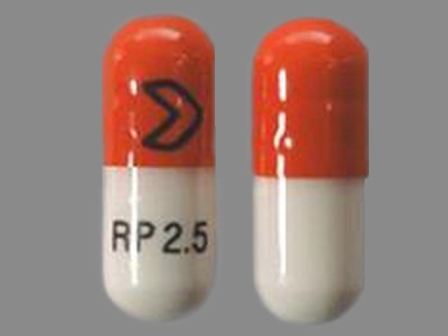
0054-0107 Jun 18, 2008
Ramipril 2.5 mg Oral Capsule by Roxane Laboratories, Inc
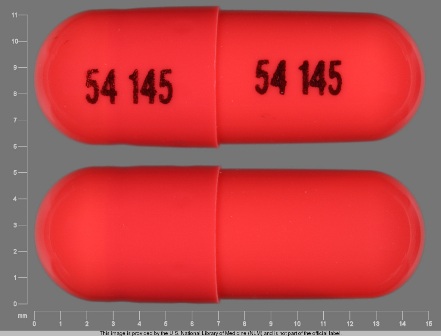
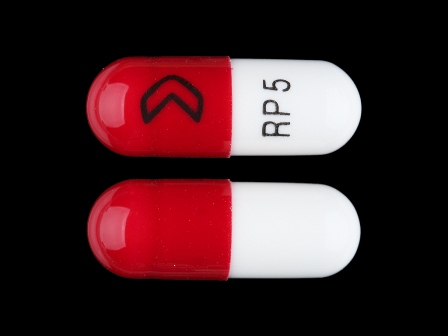
0054-0108 Jun 18, 2008
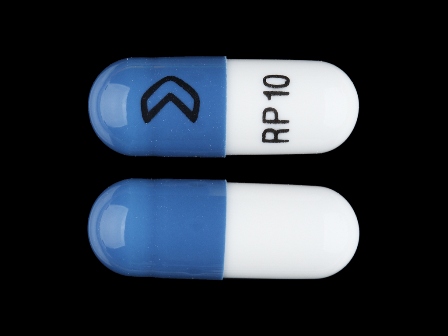
Ramipril 5 mg Oral Capsule by Roxane Laboratories, Inc
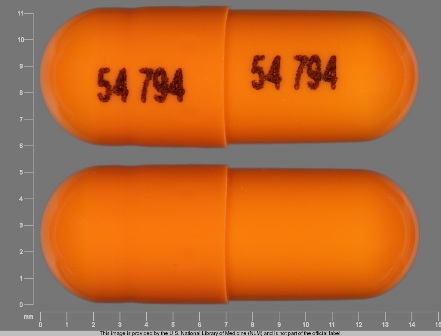
0054-0109 Jun 18, 2008
Ramipril 10 mg Oral Capsule by Roxane Laboratories, Inc
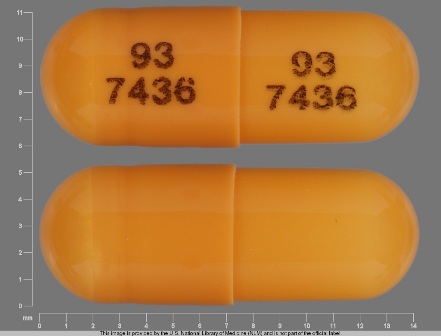
0093-7436 Jun 24, 2008
Ramipril 2.5 mg Oral Capsule by Teva Pharmaceuticals USA Inc
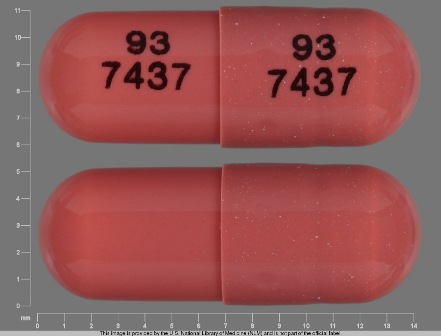
0093-7437 Jun 24, 2008
Ramipril 5 mg Oral Capsule by Teva Pharmaceuticals USA Inc
0093-7438 Jun 24, 2008
Ramipril 10 mg Oral Capsule by Teva Pharmaceuticals USA Inc
16252-570 May 05, 2008
Ramipril 1.25 mg Oral Capsule by Cobalt Laboratories
16252-571 Jan 14, 2008
Ramipril 2.5 mg Oral Capsule by Cobalt Laboratories
16252-572 Dec 26, 2007
Ramipril 5 mg Oral Capsule by Cobalt Laboratories
16252-573 Dec 26, 2007
Ramipril 10 mg Oral Capsule by Cobalt Laboratories
31722-272 Jan 01, 2011
Ramipril 2.5 mg Oral Capsule by Camber Pharmaceuticals
31722-273 Jan 01, 2011
Ramipril 5 mg Oral Capsule by Camber Pharmaceuticals
31722-274 Jan 01, 2011
Ramipril 10 mg Oral Capsule by Camber Pharmaceuticals
50436-9981 Jun 24, 2008
Ramipril 10 mg Oral Capsule by Unit Dose Services
60429-038 Jun 18, 2008
Ramipril 1.25 mg Oral Capsule by Golden State Medical Supply, Inc.
60429-039 Jun 18, 2008
Ramipril 2.5 mg Oral Capsule by Golden State Medical Supply, Inc.
60429-040 Jun 18, 2008
Ramipril 5 mg Oral Capsule by Golden State Medical Supply, Inc.
60429-041 Jun 18, 2008
Ramipril 10 mg Oral Capsule by Golden State Medical Supply, Inc.
60505-2875 Jun 20, 2008
Ramipril 1.25 mg Oral Capsule by Apotex Corp.
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .