Pyrazinamide - NDC Database
25 records found
Page: 1 Start a Discussion
60687-138 Oct 24, 2016
Pyrazinamide 500 mg Oral Tablet by American Health Packaging
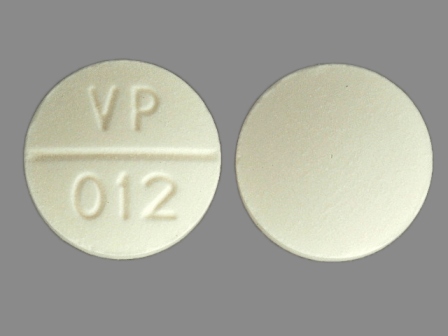
61748-012 Apr 01, 1995
Pza 500 mg Oral Tablet by Versapharm Incorporated
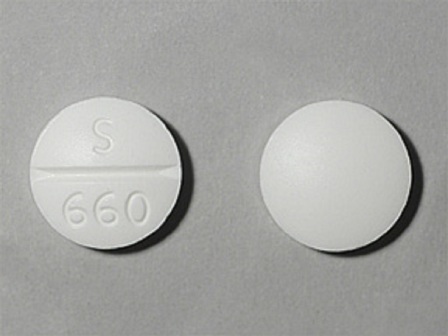
67253-660 Jun 03, 1971
Pza 500 mg Oral Tablet by Dava Pharmaceuticals, Inc.
0904-6696 Jun 03, 1971
Pyrazinamide 500 mg Oral Tablet by Major Pharmaceuticals
24236-687 May 21, 2008
Pyrazinamide 500 mg Oral Tablet by Remedyrepack Inc.
24236-763 Jun 29, 2015
Pza 500 mg Oral Tablet by Remedyrepack Inc.
49349-344 Jun 30, 2011
Pza 500 mg Oral Tablet by Remedyrepack Inc.
50090-0520 Apr 01, 1995
Pyrazinamide 500 mg Oral Tablet by A-s Medication Solutions
50090-0521 Jun 03, 1971
Pyrazinamide 500 mg Oral Tablet by A-s Medication Solutions
52125-013 Feb 26, 2013
Pza 500 mg Oral Tablet by Remedyrepack Inc.
54569-3950 Apr 01, 1995
Pyrazinamide 500 mg Oral Tablet by A-s Medication Solutions
55695-026 Apr 01, 1995
Pyrazinamide 500 mg Oral Tablet by Department of State Health Services, Pharmacy Branch
58118-0004 Jun 03, 1971
Pyrazinamide 500 mg Oral Tablet by Clinical Solutions Wholesale, LLC
70518-0085 Jan 10, 2017
Pyrazinamide 500 mg Oral Tablet by Remedyrepack Inc.
70518-2534 Jan 10, 2020
Pyrazinamide 500 mg Oral Tablet by Remedyrepack Inc.
0054-0812 Aug 30, 2024
Pyrazinamide 500 mg Oral Tablet by Hikma Pharmaceuticals USA Inc
10135-735 Mar 01, 2022
Pyrazinamide 500 mg Oral Tablet by Marlex Pharmaceuticals, Inc
33342-447 Jul 27, 2020
Pyrazinamide 500 mg Oral Tablet by Macleods Pharmaceuticals Limited
46672-108 Jun 30, 1992
Pyrazinamide 500 mg Oral Tablet by Mikart, Inc.
50090-5963 May 11, 2021
Pyrazinamide 500 mg Oral Tablet by A-s Medication Solutions
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .