Phenytoin Sodium - NDC Database
78 records found
Page: 1 Start a Discussion
0378-1560 Jan 05, 1999
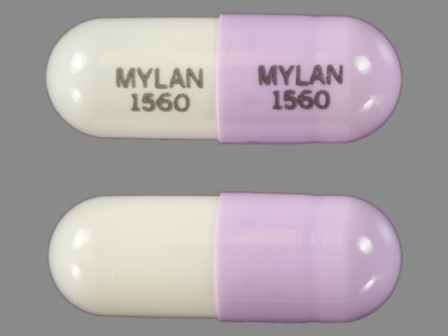
Dph Sodium 100 mg Extended Release Capsule by Mylan Pharmaceuticals Inc.
51672-4111 Sep 05, 2006
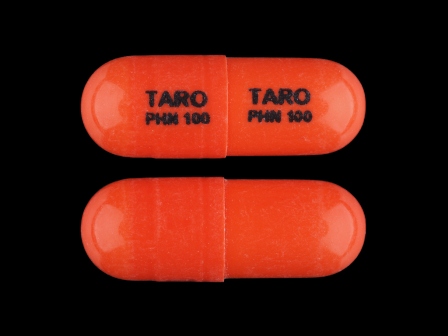
Dph Sodium 100 mg Extended Release Capsule by Taro Pharmaceuticals U.S.a., Inc.
62756-299 Jun 30, 2008
Dph Sodium 200 mg Extended Release Capsule by Sun Pharmaceutical Industries Limited
0615-1343 Nov 20, 2009
Dph Sodium 100 mg Extended Release Capsule by Ncs Healthcare of Ky, Inc Dba Vangard Labs
0615-8020 Sep 05, 2006
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Ncs Healthcare of Ky, Inc Dba Vangard Labs
21695-167 Sep 05, 2006
Dph Sodium 100 mg Extended Release Capsule by Rebel Distributors Corp
50090-3499 Sep 05, 2006
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by A-s Medication Solutions
50090-3539 Sep 05, 2006
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by A-s Medication Solutions
51079-905 Sep 12, 2012
Dph Sodium 100 mg Extended Release Capsule by Mylan Institutional Inc.
52125-160 Mar 20, 2013
Dph Sodium 100 mg Extended Release Capsule by Remedyrepack Inc.
52125-685 Aug 27, 2013
Dph Sodium 100 mg Extended Release Capsule by Remedyrepack Inc.
54868-5534 Jul 12, 2007
Dph Sodium 100 mg Extended Release Capsule by Physicians Total Care, Inc.
55154-3027 Sep 05, 2006
Dph Sodium 100 mg Extended Release Capsule by Cardinal Health
55154-5496 Jan 28, 2011
Dph Sodium 100 mg Extended Release Capsule by Cardinal Health
55154-6217 Nov 20, 2009
Dph Sodium 100 mg Extended Release Capsule by Cardinal Health
58118-4111 Mar 07, 2019
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Clinical Solutions Wholesale, LLC
63629-2552 Jul 07, 2011
Dph Sodium 100 mg Extended Release Capsule by Bryant Ranch Prepack
64725-4111 Sep 05, 2006
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Tya Pharmaceuticals
67046-585 Apr 06, 2010
Dph Sodium 100 mg Extended Release Capsule by Contract Pharmacy Services-pa
67544-517 Jan 05, 1999
Phenytoin Sodium 100 mg Oral Capsule, Extended Release by Aphena Pharma Solutions - Tennessee, LLC
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .