Ondansetron Hydrochloride - NDC Database

Ondansetron 4 mg (Ondansetron Hydrochloride Dihydrate 5 mg) Oral Tablet by Teva Pharmaceuticals USA Inc

Ondansetron 8 mg (As Ondansetron Hydrochloride Dihydrate 10 mg) Oral Tablet by Teva Pharmaceuticals USA Inc

Ondansetron 4 mg (Ondansetron Hydrochloride Dihydrate 5 mg) Oral Tablet by Sandoz Inc

Ondansetron 8 mg (As Ondansetron Hydrochloride Dihydrate 10 mg) Oral Tablet by Sandoz Inc
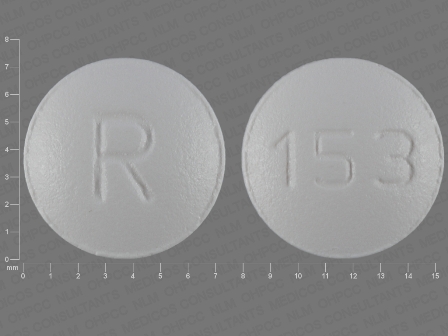
Ondansetron 4 mg (Ondansetron Hydrochloride Dihydrate 5 mg) Oral Tablet by Dr.reddy's Laboratories Limited

Ondansetron 8 mg (As Ondansetron Hydrochloride Dihydrate 10 mg) Oral Tablet by Dr.reddy's Laboratories Limited

Ondansetron 4 mg (Ondansetron Hydrochloride Dihydrate 5 mg) Oral Tablet by Sun Pharmaceutical Industries Limited

Ondansetron 8 mg (As Ondansetron Hydrochloride Dihydrate 10 mg) Oral Tablet by Sun Pharmaceutical Industries Limited

Ondansetron 4 mg (Ondansetron Hydrochloride Dihydrate 5 mg) Oral Tablet by Ascend Laboratories, LLC
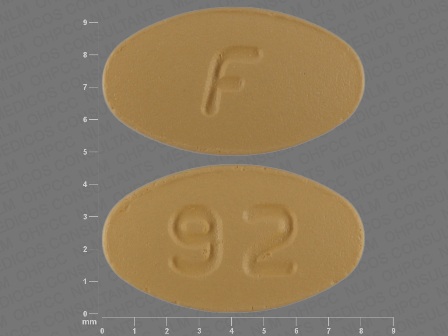
Ondansetron 8 mg (As Ondansetron Hydrochloride Dihydrate 10 mg) Oral Tablet by American Health Packaging

Ondansetron 4 mg (Ondansetron Hydrochloride Dihydrate 5 mg) Oral Tablet by Kaiser Foundation Hospitals
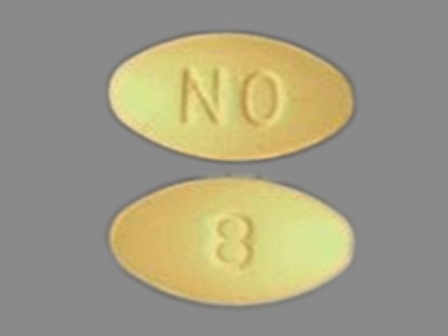
Ondansetron 8 mg (As Ondansetron Hydrochloride Dihydrate 10 mg) Oral Tablet by Kaiser Foundation Hospitals

Ondansetron Hydrochloride 4 mg Oral Tablet, Film Coated by Kaiser Foundation Hospitals

Ondansetron Hydrochloride 8 mg Oral Tablet, Film Coated by Kaiser Foundation Hospitals

Ondansetron Hydrochloride 4 mg Oral Tablet, Film Coated by Ncs Healthcare of Ky, Inc Dba Vangard Labs

Ondansetron Hydrochloride 4 mg Oral Tablet, Film Coated by Major Pharmaceuticals

Ondansetron Hydrochloride 8 mg Oral Tablet, Film Coated by Major Pharmaceuticals

Ondansetron Hydrochloride 8 mg Oral Tablet, Film Coated by Blenheim Pharmacal, Inc.

Ondansetron 4 mg (Ondansetron Hydrochloride Dihydrate 5 mg) Oral Tablet by Rebel Distributors Corp
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.