Omeprazole - NDC Database (Page 2)
629 records found
Start a Discussion
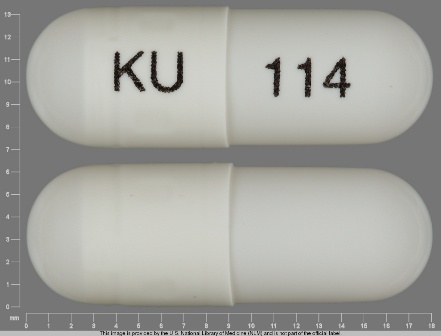
62175-136 Jan 23, 2009
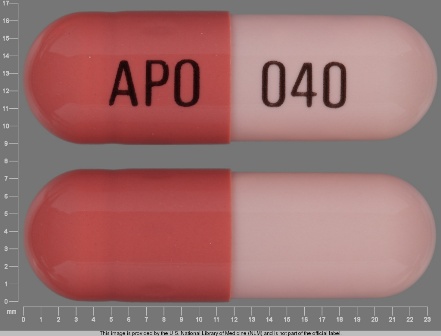
Omeprazole 40 mg Delayed Release Capsule by Kremers Urban Pharmaceuticals Inc.
63187-170 Jan 21, 2009
Omeprazole 40 mg Oral Capsule, Delayed Release by Proficient Rx Lp
67544-510 Nov 04, 2002
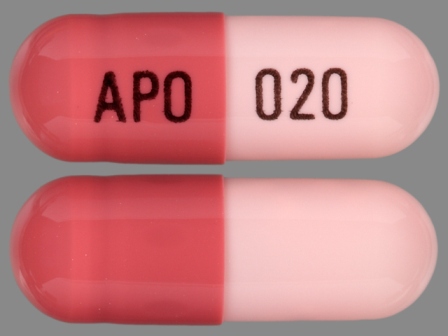
Omeprazole 20 mg Delayed Release Capsule by Aphena Pharma Solutions - Tennessee, Inc.
68084-128 Dec 07, 2009
Omeprazole 20 mg Delayed Release Capsule by American Health Packaging
68084-466 Apr 12, 2011
Omeprazole 40 mg Delayed Release Capsule by American Health Packaging
68462-231 Oct 31, 2014
Omeprazole 20 mg Oral Capsule, Delayed Release by Glenmark Generics Inc., USA
69618-047 Aug 16, 2018
Omeprazole 20 mg Oral Capsule by Reliable 1 Laboratories LLC
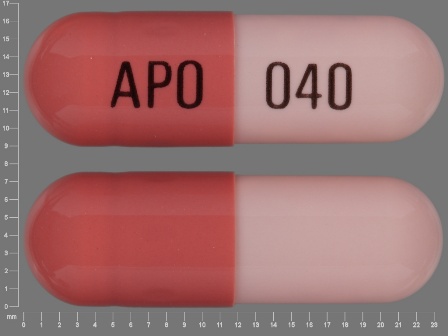
0179-0016 Aug 01, 2009
Omeprazole 40 mg Delayed Release Capsule by Kaiser Foundation Hospitals
0179-1804 Jun 02, 2006
Omeprazole 10 mg Delayed Release Capsule by Kaiser Foundation Hospitals
0615-2302 Oct 22, 2007
Omeprazole 40 mg Delayed Release Capsule by Ncs Healthcare of Ky, Inc Dba Vangard Labs
0615-2305 Oct 22, 2007
Omeprazole 20 mg Delayed Release Capsule by Ncs Healthcare of Ky, Inc Dba Vangard Labs
0615-7933 Jan 28, 2003
Omeprazole 20 mg Oral Capsule, Delayed Release by Ncs Healthcare of Ky, Inc Dba Vangard Labs
0781-2790 Jan 28, 2003
Omeprazole 20 mg Oral Capsule, Delayed Release by Sandoz Inc
10544-295 Sep 30, 2013
Omeprazole 20 mg Oral Capsule, Delayed Release by Blenheim Pharmacal, Inc.
10544-299 Sep 30, 2013
Omeprazole 40 mg Oral Capsule, Delayed Release by Blenheim Pharmacal, Inc.
10544-878 Oct 15, 2014
Omeprazole 20 mg Oral Capsule, Delayed Release by Blenheim Pharmacal, Inc.
10544-895 Feb 25, 2015
Omeprazole 40 mg Oral Capsule, Delayed Release by Blenheim Pharmacal, Inc.
10544-898 Mar 10, 2015
Omeprazole 20 mg Oral Capsule, Delayed Release by Blenheim Pharmacal, Inc.
10544-972 Feb 09, 2016
Omeprazole 20 mg Oral Capsule, Delayed Release by Blenheim Pharmacal, Inc.
16714-715 Oct 25, 2019
Omeprazole 20 mg Oral Capsule, Delayed Release by Northstar Rx LLC
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .