Irbesartan - NDC Database
158 records found
Page: 1 Start a Discussion
0054-0252 Oct 15, 2012
Irbesartan 300 mg Oral Tablet by Roxane Laboratories, Inc.
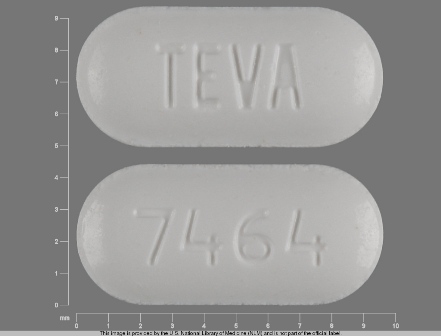
0093-7464 Mar 30, 2012
Irbesartan 75 mg Oral Tablet by Teva Pharmaceuticals USA Inc
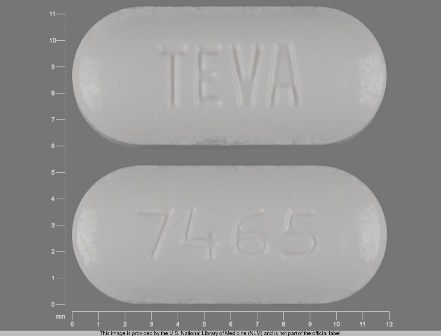
0093-7465 Mar 30, 2012
Irbesartan 150 mg Oral Tablet by Teva Pharmaceuticals USA Inc
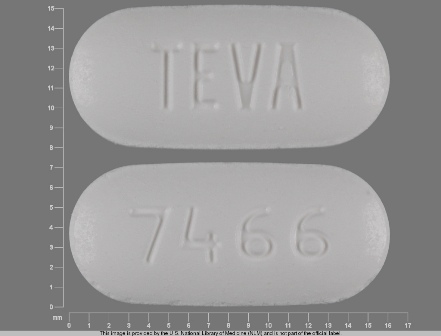
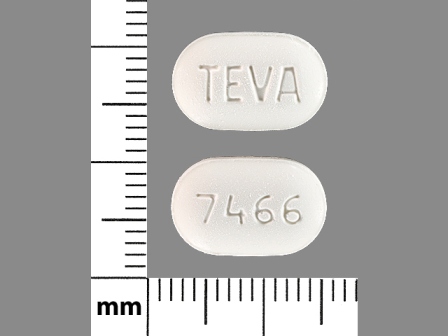
0093-7466 Mar 30, 2012
Irbesartan 300 mg Oral Tablet by Teva Pharmaceuticals USA Inc
0093-7514 May 10, 2013
Irbesartan 300 mg Oral Tablet by Teva Pharmaceuticals USA Inc
0378-2022 Sep 28, 2012
Irbesartan 75 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0955-1040 Sep 30, 2019
Irbesartan 75 mg Oral Tablet by Winthrop U.S.
0955-1041 Sep 30, 2019
Irbesartan 150 mg Oral Tablet by Winthrop U.S.
0955-1042 Sep 30, 2019
Irbesartan 300 mg Oral Tablet by Winthrop U.S.
31722-731 Sep 27, 2012
Irbesartan 300 mg Oral Tablet by Camber Pharmaceuticals, Inc.
43547-277 Sep 27, 2012
Irbesartan 75 mg Oral Tablet by Solco Healthcare U.S., LLC
43547-278 Sep 27, 2012
Irbesartan 150 mg Oral Tablet by Solco Healthcare U.S., LLC
43547-279 Sep 27, 2012
Irbesartan 300 mg Oral Tablet by Solco Healthcare U.S., LLC
68180-412 Oct 23, 2012
Irbesartan 300 mg Oral Tablet by Lupin Pharmaceuticals, Inc.
50090-1613 Oct 15, 2012
Irbesartan 300 mg Oral Tablet by A-s Medication Solutions
50090-2017 Sep 27, 2012
Irbesartan 300 mg Oral Tablet by A-s Medication Solutions
50090-2642 Sep 27, 2012
Irbesartan 300 mg Oral Tablet by A-s Medication Solutions
50090-5055 Sep 30, 2019
Irbesartan 300 mg Oral Tablet, Film Coated by A-s Medication Solutions
50268-442 Aug 08, 2023
Irbesartan 300 mg Oral Tablet by Avpak
57297-412 Oct 23, 2012
Irbesartan 300 mg Oral Tablet by Lupin Limited
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .