Ibandronate - NDC Database
18 records found
Page:

Ibandronic Acid 150 mg (As Ibandronate Sodium 168.75 mg) Oral Tablet by Watson Laboratories, Inc.
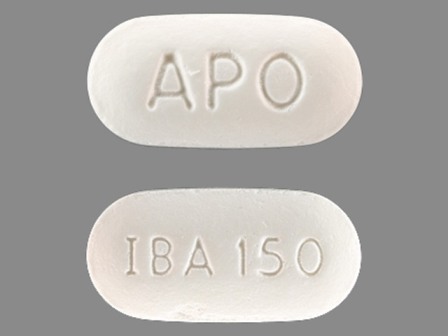
Ibandronic Acid 150 mg (As Ibandronate Sodium 168.75 mg) Oral Tablet by Apotex Corp

Ibandronate Sodium 150 mg Oral Tablet, Film Coated by Golden State Medical Supply, Inc.
0378-5215 Mar 19, 2012
Ibandronic Acid 150 mg (As Ibandronate Sodium 168.75 mg) Oral Tablet by Mylan Pharmaceuticals Inc.
Ibandronic Acid 150 mg (As Ibandronate Sodium 168.75 mg) Oral Tablet by Mylan Pharmaceuticals Inc.
16729-274 Jan 01, 2024
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Accord Healthcare Inc.
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Accord Healthcare Inc.
23155-162 Sep 04, 2014
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Heritage Pharmaceuticals Inc.
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Heritage Pharmaceuticals Inc.
25021-827 Sep 02, 2014
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Sagent Pharmaceuticals
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Sagent Pharmaceuticals
33342-150 Nov 01, 2017
Ibandronate Sodium 150 mg Oral Tablet, Film Coated by Macleods Pharmaceuticals Limited
Ibandronate Sodium 150 mg Oral Tablet, Film Coated by Macleods Pharmaceuticals Limited
51224-008 Jun 30, 2022
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Tagi Pharma, Inc.
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Tagi Pharma, Inc.
55111-575 Jun 21, 2012
Ibandronic Acid 150 mg (As Ibandronate Sodium 168.75 mg) Oral Tablet by Dr. Reddy's Laboratories Limited
Ibandronic Acid 150 mg (As Ibandronate Sodium 168.75 mg) Oral Tablet by Dr. Reddy's Laboratories Limited
55150-191 Aug 19, 2015
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Auromedics Pharma LLC
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Auromedics Pharma LLC
62756-218 Feb 15, 2014
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Sun Pharmaceutical Industries Limited
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Sun Pharmaceutical Industries Limited
65862-237 Mar 11, 2016
Ibandronate Sodium 150 mg Oral Tablet, Film Coated by Aurobindo Pharma Limited
Ibandronate Sodium 150 mg Oral Tablet, Film Coated by Aurobindo Pharma Limited
67457-524 Sep 02, 2014
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Mylan Institutional LLC
Ibandronate Sodium 3 mg/3ml Intravenous Injection, Solution by Mylan Institutional LLC
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
