Hyoscyamine Sulfate - NDC Database
78 records found
Page: 1 Start a Discussion
0574-0246 Aug 06, 2012
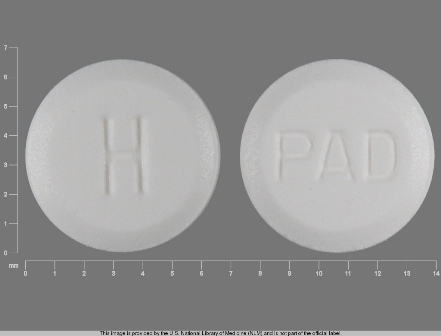
Hyoscyamine Sulfate 0.125 mg Oral Tablet by Paddock Laboratories, LLC
0574-0247 Aug 06, 2012
Hyoscyamine Sulfate 0.125 mg Chewable Tablet by Paddock Laboratories, LLC
0574-0250 Aug 06, 2012
Hyoscyamine Sulfate Sl 0.125 Disintegrating Sublingual Tablet by Paddock Laboratories, LLC
0574-0251 Sep 19, 2009
Hyoscyamine Sulfate 0.375 mg Biphasic (0.125 mg / 0.25 mg) 12 Hr Extended Release Tablet by Paddock Laboratories, Inc.
43199-011 Sep 21, 2009
Hyoscyamine Sulfate Sl 0.125 Disintegrating Sublingual Tablet by County Line Pharmaceuticals, LLC
43199-012 Sep 21, 2009
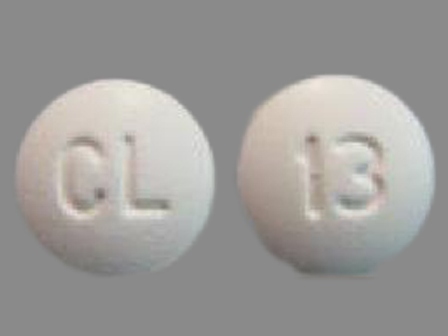
Hyoscyamine Sulfate 0.125 mg Chewable Tablet by County Line Pharmaceuticals, LLC
43199-013 Nov 05, 2009
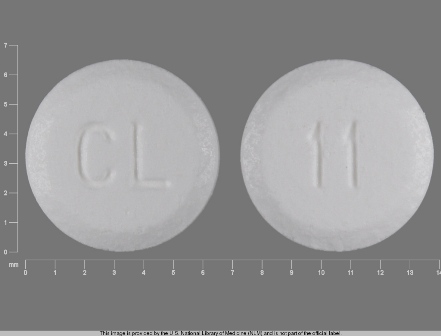
Hyoscyamine Sulfate 0.125 mg Oral Tablet by County Line Pharmaceuticals, LLC
50532-112 May 01, 2010
Hyoscyamine Sulfate 0.125 mg Oral Tablet by Franklin Pharmaceutical LLC
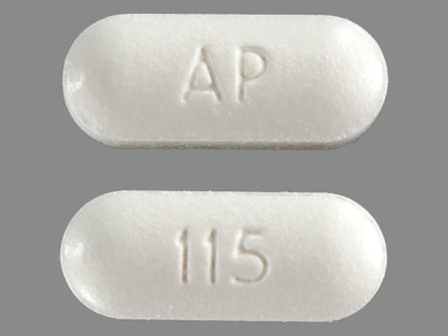
50532-115 May 01, 2010
Hyoscyamine Sulfate 0.375 mg Biphasic (0.125 mg / 0.25 mg) 12 Hr Extended Release Tablet by Franklin Pharmaceutical LLC
57629-203 Jan 01, 2011
Hyoscyamine Sulfate .375 mg Oral Tablet by Kmr Pharmaceuticals LLC
76439-307 Sep 04, 2011
Hyoscyamine Sulfate 0.125 mg Chewable Tablet by Virtus Pharmaceuticals
76439-308 Sep 04, 2011
Hyoscyamine Sulfate 0.125 mg Oral Tablet by Virtus Pharmaceuticals
21695-442 Nov 05, 2009
Hyoscyamine Sulfate 0.125 mg Oral Tablet by Rebel Distributors Corp
21695-926 Sep 21, 2009
Hyoscyamine Sulfate Sl 0.125 Disintegrating Sublingual Tablet by Rebel Distributors Corp
43063-321 Sep 21, 2009
Hyoscyamine Sulfate Sl 0.125 Disintegrating Sublingual Tablet by Pd-rx Pharmaceuticals, Inc.
53002-1135 Nov 05, 2009
Hyoscyamine Sulfate .125 mg Oral Tablet by Rpk Pharmaceuticals, Inc.
53217-063 Nov 05, 2009
Hyoscyamine Sulfate .125 mg/1 Oral Tablet by Aidarex Pharmaceuticals LLC
58118-0013 Nov 05, 2009
Hyoscyamine Sulfate 0.375 mg Biphasic (0.125 mg / 0.25 mg) 12 Hr Extended Release Tablet by Clinical Solutions Wholesale
61919-759 Nov 06, 2017
Hyoscyamine Sulfate .125 mg Oral Tablet by Direct Rx
63187-058 Sep 21, 2009
Hyoscyamine Sulfate .125 mg Oral; Sublingual Tablet by Proficient Rx Lp
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .