Granisetron - NDC Database

Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
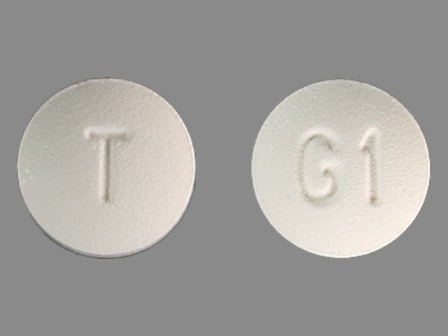
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Taro Pharmaceuticals U.S.a., Inc.
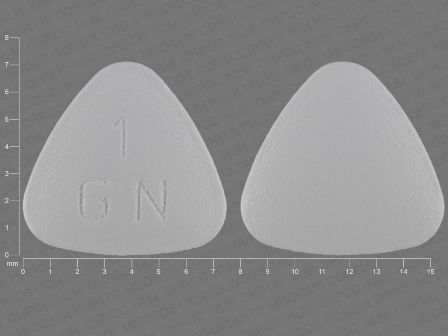
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Breckenridge Pharmaceutical, Inc.

Granisetron Hydrochloride 1 mg Oral Tablet, Film Coated by Natco Pharma Limited

Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Ascend Laboratories, LLC

Granisetron Hydrochloride 1 mg Oral Tablet, Film Coated by Avera Mckennan Hospital
0054-0143 May 16, 2008
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Roxane Laboratories, Inc
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Roxane Laboratories, Inc
0143-9744 Dec 23, 2009
Granisetron Hydrochloride 1 mg/ml Intravenous Solution by West-ward Pharmaceutical Corp
Granisetron Hydrochloride 1 mg/ml Intravenous Solution by West-ward Pharmaceutical Corp
0143-9745 Dec 23, 2009
Granisetron Hydrochloride 4 mg/4ml Intravenous Solution by West-ward Pharmaceutical Corp
Granisetron Hydrochloride 4 mg/4ml Intravenous Solution by West-ward Pharmaceutical Corp
16714-221 Apr 29, 2008
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Northstar Rx LLC
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Northstar Rx LLC
25021-778 May 31, 2013
Granisetron Hydrochloride .1 mg/ml Intravenous Injection by Sagent Pharmaceuticals
Granisetron Hydrochloride .1 mg/ml Intravenous Injection by Sagent Pharmaceuticals
25021-779 Dec 01, 2010
Granisetron Hydrochloride 1 mg/ml Intravenous Injection by Sagent Pharmaceuticals
Granisetron Hydrochloride 1 mg/ml Intravenous Injection by Sagent Pharmaceuticals
25021-781 Dec 01, 2010
Granisetron Hydrochloride 1 mg/ml Intravenous Injection by Sagent Pharmaceuticals
Granisetron Hydrochloride 1 mg/ml Intravenous Injection by Sagent Pharmaceuticals
51079-472 Jul 23, 2010
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Udl Laboratories, Inc.
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet by Udl Laboratories, Inc.
55150-175 Jul 06, 2016
Granisetron Hydrochloride 1 mg/ml Intravenous Injection, Solution by Auromedics Pharma LLC
Granisetron Hydrochloride 1 mg/ml Intravenous Injection, Solution by Auromedics Pharma LLC
55150-176 Jul 06, 2016
Granisetron Hydrochloride 4 mg/4ml Intravenous Injection, Solution by Auromedics Pharma LLC
Granisetron Hydrochloride 4 mg/4ml Intravenous Injection, Solution by Auromedics Pharma LLC
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.