Fexofenadine - NDC Database
507 records found
Page: 1 Start a Discussion
0378-0781 Aug 25, 2011
Fexofenadine Hydrochloride 60 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0378-0782 Aug 25, 2011
Fexofenadine Hydrochloride 180 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0904-6214 Apr 14, 2011
Fexofenadine Hydrochloride 180 mg Oral Tablet by Major Pharmaceuticals
41167-4120 Mar 03, 2011
Allegra 180 mg Oral Tablet by Chattem, Inc.
43353-244 Jul 01, 2010
Fexofenadine Hydrochloride 180 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, Inc.
45802-425 Aug 08, 2011
Fexofenadine Hydrochloride 60 mg Oral Tablet by Perrigo New York Inc
45802-571 Apr 22, 2011
Fexofenadine Hydrochloride 180 mg Oral Tablet by Perrigo New York Inc
51079-529 Aug 27, 2010
Fexofenadine Hydrochloride 60 mg Oral Tablet by Udl Laboratories, Inc.
60429-388 Mar 20, 2014
Fexofenadine Hydrochloride 60 mg Oral Tablet, Film Coated by Golden State Medical Supply, Inc.
60429-389 Mar 20, 2014
Fexofenadine Hydrochloride 180 mg Oral Tablet, Film Coated by Golden State Medical Supply, Inc.
0113-0425 Sep 14, 2011
Fexofenadine Hydrochloride 60 mg Oral Tablet by L Perrigo Company
0113-0571 Apr 14, 2011
Fexofenadine Hydrochloride 180 mg Oral Tablet by L Perrigo Company
0113-7425 Apr 10, 2019
Basic Care Allergy 60 mg Oral Tablet, Film Coated by L. Perrigo Company
0113-7571 May 09, 2018
Basic Care Allergy 180 mg Oral Tablet, Film Coated by L. Perrigo Company
0363-0425 Aug 22, 2011
Wal-fex 60 mg Oral Tablet by Walgreen Company
0363-0571 Apr 14, 2011
Wal-fex 180 mg Oral Tablet by Walgreen Company
0363-0600 Mar 06, 2014
Wal Fex 24 Hour Allergy 180 mg/1 Oral Tablet, Film Coated by Walgreen Company
0363-0903 Aug 22, 2011
Wal-fex 60 mg Oral Tablet by Walgreen Company
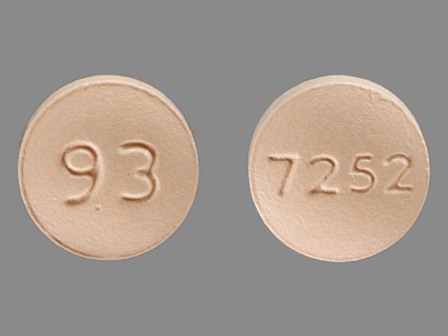
0480-7252 Sep 17, 2010
Fexofenadine Hydrochloride 60 mg Oral Tablet by Teva Pharmaceutical Industries Ltd.
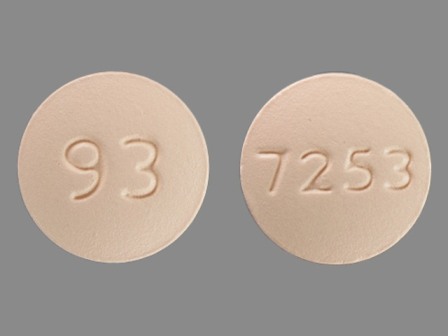
0480-7253 Sep 17, 2010
Fexofenadine Hydrochloride 180 mg Oral Tablet by Teva Pharmaceutical Industries Ltd.
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .