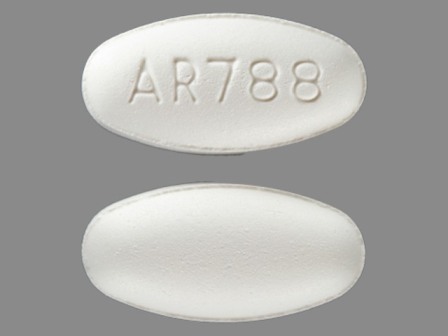
Fenofibric Acid - NDC Database
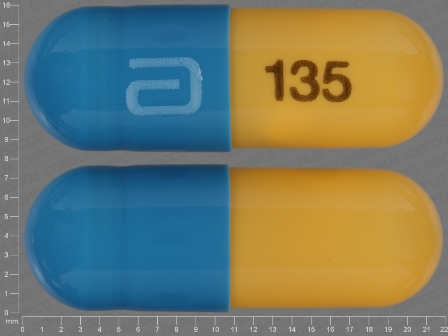
Fenofibric Acid 135 mg Delayed Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
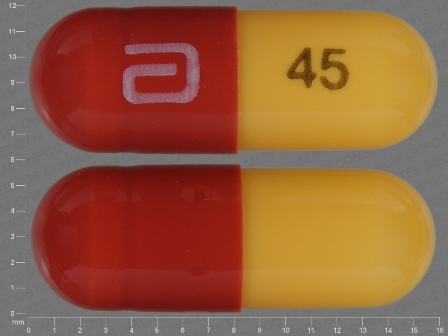
Fenofibric Acid 45 mg/1 Oral Capsule, Delayed Release by Zydus Pharmaceuticals USA Inc
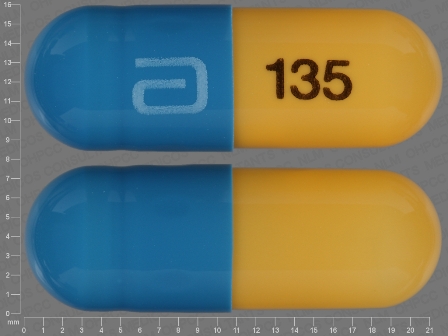
Fenofibric Acid 135 mg/1 Oral Capsule, Delayed Release by Zydus Pharmaceuticals USA Inc
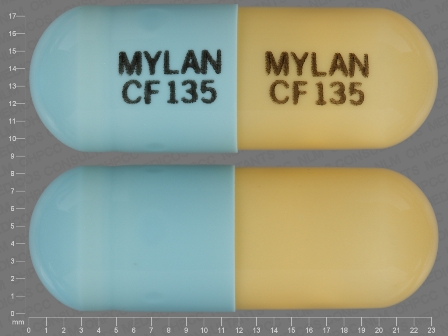
Fenofibric Acid 135 mg/1 Oral Capsule, Delayed Release Pellets by Mylan Institutional Inc.
0115-1324 Jun 01, 2024
Fenofibric Acid 45 mg Oral Capsule, Delayed Release by Amneal Pharmaceuticals of New York LLC
Fenofibric Acid 45 mg Oral Capsule, Delayed Release by Amneal Pharmaceuticals of New York LLC
0115-1325 Jun 01, 2024
Fenofibric Acid 135 mg Oral Capsule, Delayed Release by Amneal Pharmaceuticals of New York LLC
Fenofibric Acid 135 mg Oral Capsule, Delayed Release by Amneal Pharmaceuticals of New York LLC
0115-1459 Dec 15, 2008
Fenofibric Acid 45 mg Delayed Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
Fenofibric Acid 45 mg Delayed Release Capsule by Global Pharmaceuticals, Division of Impax Laboratories Inc.
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.