Extra Strength Pain Relief - NDC Database
Extra Strength Pain Relief 500 mg Oral Tablet, Coated by Chain Drug Consortium, LLC
15127-857 Nov 17, 1992
Apap 250 mg / Asa 250 mg / Caffeine 65 mg Oral Tablet by Stephen L. Lafrance Pharmacy, Inc.
Apap 250 mg / Asa 250 mg / Caffeine 65 mg Oral Tablet by Stephen L. Lafrance Pharmacy, Inc.
33992-0519 May 10, 2004
Azacitidine 25 mg/ml Injectable Suspension by Greenbrier International, Inc.
Azacitidine 25 mg/ml Injectable Suspension by Greenbrier International, Inc.
36800-414 Jul 11, 2016
Extra Strength Pain Relief 500 mg Oral Capsule, Liquid Filled by Topco Associates LLC
Extra Strength Pain Relief 500 mg Oral Capsule, Liquid Filled by Topco Associates LLC
49738-024 Jun 01, 2016
Extra Strength Pain Relief 500 mg Oral Capsule, Liquid Filled by Kmart Corporation
Extra Strength Pain Relief 500 mg Oral Capsule, Liquid Filled by Kmart Corporation
51013-128 Jul 12, 2016
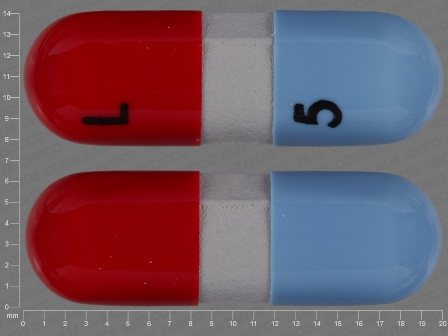
Extra Strength Pain Relief 500 mg Oral Capsule, Liquid Filled by Puracap Pharmaceutical LLC
Extra Strength Pain Relief 500 mg Oral Capsule, Liquid Filled by Puracap Pharmaceutical LLC
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.