Duloxetine - NDC Database (Page 4)
416 records found
Start a Discussion
59651-280 Dec 11, 2013
Duloxetine 30 mg Oral Capsule, Delayed Release by Aurobindo Pharma Limited
59651-282 Dec 11, 2013
Duloxetine 60 mg Oral Capsule, Delayed Release by Aurobindo Pharma Limited
61786-568 Jan 29, 2016
Duloxetine 30 mg Oral Capsule, Delayed Release by Remedyrepack Inc.
61786-628 May 13, 2016
Duloxetine Delayed-release 30 mg Oral Capsule, Delayed Release Pellets by Remedyrepack Inc.
61786-683 May 10, 2016
Duloxetine 60 mg Oral Capsule, Delayed Release Pellets by Remedyrepack Inc.
61786-684 May 10, 2016
Duloxetine 20 mg Oral Capsule, Delayed Release by Remedyrepack Inc.
61786-935 Oct 12, 2016
Duloxetine 30 mg Oral Capsule, Delayed Release by Remedyrepack Inc.
61919-422 Aug 19, 2019
Duloxetine 20 mg Oral Capsule, Delayed Release Pellets by Direct Rx
62034-021 Apr 01, 2016
Duloxetine 60 mg Oral Capsule, Delayed Release Pellets by Blenheim Pharmacal, Inc.
62034-029 Apr 01, 2016
Duloxetine 30 mg Oral Capsule, Delayed Release Pellets by Blenheim Pharmacal, Inc.
63187-612 Dec 11, 2013
Duloxetine 30 mg Oral Capsule, Delayed Release by Proficient Rx Lp
63187-666 Dec 11, 2013
Duloxetine 60 mg Oral Capsule, Delayed Release by Proficient Rx Lp
63187-702 Jun 11, 2014
Duloxetine 30 mg Oral Capsule, Delayed Release Pellets by Proficient Rx Lp
63187-720 Jun 11, 2014
Duloxetine 20 mg Oral Capsule, Delayed Release Pellets by Proficient Rx Lp
63187-735 Jun 11, 2014
Duloxetine 60 mg Oral Capsule, Delayed Release Pellets by Proficient Rx Lp
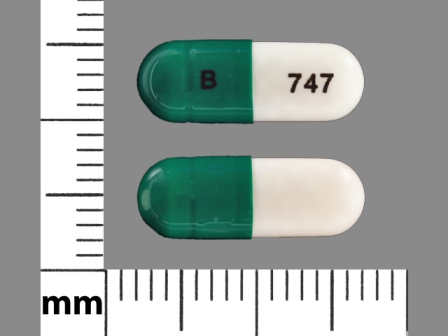
64725-0747 Jun 11, 2014
Duloxetine 30 mg Oral Capsule, Delayed Release Pellets by Tya Pharmaceuticals
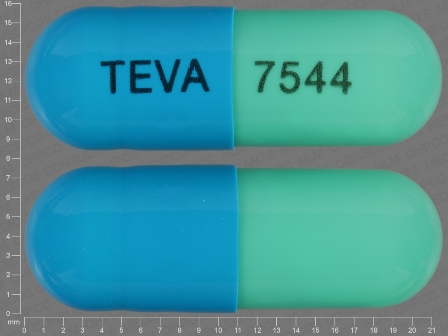
64725-7544 Dec 11, 2013
Duloxetine 60 mg Oral Capsule, Delayed Release by Tya Pharmaceuticals
65862-451 Dec 11, 2013
Duloxetine 20 mg Oral Capsule, Delayed Release by Aurobindo Pharma Limited
65862-452 Dec 11, 2013
Duloxetine 30 mg Oral Capsule, Delayed Release by Aurobindo Pharma Limited
65862-453 Dec 11, 2013
Duloxetine 60 mg Oral Capsule, Delayed Release by Aurobindo Pharma Limited
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .