Desvenlafaxine Succinate - NDC Database
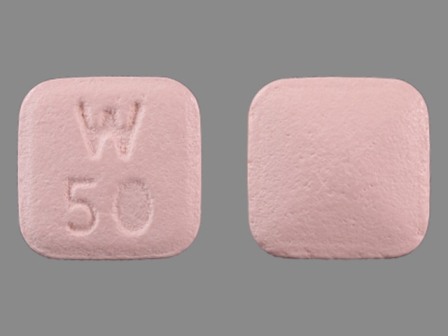
24 Hr Pristiq 50 mg Extended Release Tablet by Wyeth Pharmaceuticals Inc., a Subsidiary of Pfizer Inc.

24 Hr Pristiq 100 mg Extended Release Tablet by Wyeth Pharmaceuticals Inc., a Subsidiary of Pfizer Inc.

24 Hr Pristiq 50 mg Extended Release Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC

24 Hr Pristiq 100 mg Extended Release Tablet by Lake Erie Medical Dba Quality Care Products LLC

Desvenlafaxine Succinate 50 mg Oral Tablet, Extended Release by A-s Medication Solutions

Desvenlafaxine Succinate 50 mg Oral Tablet, Extended Release by Greenstone LLC

Desvenlafaxine Succinate 100 mg Oral Tablet, Extended Release by Greenstone LLC
0008-1210 Apr 01, 2015
Pristiq 25 mg Oral Tablet, Extended Release by Wyeth Pharmaceuticals Inc., a Subsidiary of Pfizer Inc.
Pristiq 25 mg Oral Tablet, Extended Release by Wyeth Pharmaceuticals Inc., a Subsidiary of Pfizer Inc.
33342-388 Jul 30, 2020
Desvenlafaxine Succinate 50 mg Oral Tablet, Extended Release by Macleods Pharmaceuticals Limited
Desvenlafaxine Succinate 50 mg Oral Tablet, Extended Release by Macleods Pharmaceuticals Limited
33342-389 Jun 16, 2018
Desvenlafaxine Succinate 100 mg Oral Tablet, Extended Release by Macleods Pharmaceuticals Limited
Desvenlafaxine Succinate 100 mg Oral Tablet, Extended Release by Macleods Pharmaceuticals Limited
50090-6894 Mar 01, 2017
Desvenlafaxine Succinate 50 mg Oral Tablet, Film Coated, Extended Release by A-s Medication Solutions
Desvenlafaxine Succinate 50 mg Oral Tablet, Film Coated, Extended Release by A-s Medication Solutions
50090-7468 Aug 25, 2022
Desvenlafaxine Succinate 25 mg Oral Tablet, Film Coated, Extended Release by A-s Medication Solutions
Desvenlafaxine Succinate 25 mg Oral Tablet, Film Coated, Extended Release by A-s Medication Solutions
51991-311 Mar 01, 2017
Desvenlafaxine 50 mg Oral Tablet, Extended Release by Breckenridge Pharmaceutical, Inc
Desvenlafaxine 50 mg Oral Tablet, Extended Release by Breckenridge Pharmaceutical, Inc
51991-312 Mar 01, 2017
Desvenlafaxine 100 mg Oral Tablet, Extended Release by Breckenridge Pharmaceutical, Inc
Desvenlafaxine 100 mg Oral Tablet, Extended Release by Breckenridge Pharmaceutical, Inc
59762-1210 Mar 01, 2017
Desvenlafaxine Succinate 25 mg Oral Tablet, Extended Release by Greenstone LLC
Desvenlafaxine Succinate 25 mg Oral Tablet, Extended Release by Greenstone LLC
61919-882 Jun 04, 2019
Desvenlafaxine ER 50 mg Oral Tablet, Film Coated, Extended Release by Direct_rx
Desvenlafaxine ER 50 mg Oral Tablet, Film Coated, Extended Release by Direct_rx
68180-592 Mar 01, 2017
Desvenlafaxine Succinate 50 mg Oral Tablet, Film Coated, Extended Release by Lupin Pharmaceuticals, Inc.
Desvenlafaxine Succinate 50 mg Oral Tablet, Film Coated, Extended Release by Lupin Pharmaceuticals, Inc.
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.