Citalopram Hydrobromide - NDC Database (Page 5)

Citalopram Hydrobromide 10 mg Oral Tablet, Film Coated by Legacy Pharmaceutical Packaging, LLC
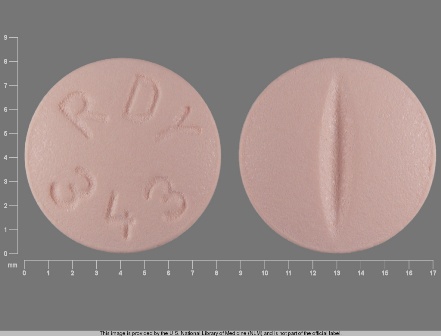
Citalopram Hydrobromide 20 mg Oral Tablet, Film Coated by Legacy Pharmaceutical Packaging, LLC

Citalopram Hydrobromide 40 mg Oral Tablet, Film Coated by Legacy Pharmaceutical Packaging, LLC

Citalopram 20 mg (As Citalopram Hydrobromide 24.99 mg) Oral Tablet by Preferred Pharmaceuticals, Inc.

Citalopram 10 mg (As Citalopram Hydrobromide 12.49 mg) Oral Tablet by Preferred Pharmaceuticals, Inc.

Citalopram Hydrobromide 20 mg Oral Tablet by Denton Pharma, Inc. Dba Northwind Pharmaceuticals
0185-0373 Oct 28, 2004
Citalopram 40 mg (As Citalopram Hydrobromide 49.98 mg) Oral Tablet by Sandoz Inc.
Citalopram 40 mg (As Citalopram Hydrobromide 49.98 mg) Oral Tablet by Sandoz Inc.
0228-2755 Jan 24, 2008
Citalopram 10 mg (As Citalopram Hydrobromide 12.49 mg) Oral Tablet by Actavis Elizabeth LLC
Citalopram 10 mg (As Citalopram Hydrobromide 12.49 mg) Oral Tablet by Actavis Elizabeth LLC
0228-2756 Jan 24, 2008
Citalopram 20 mg (As Citalopram Hydrobromide 24.99 mg) Oral Tablet by Actavis Elizabeth LLC
Citalopram 20 mg (As Citalopram Hydrobromide 24.99 mg) Oral Tablet by Actavis Elizabeth LLC
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.