Cetirizine Hydrochloride - NDC Database
244 records found
Page: 1 Start a Discussion
0378-3635 Dec 14, 2009
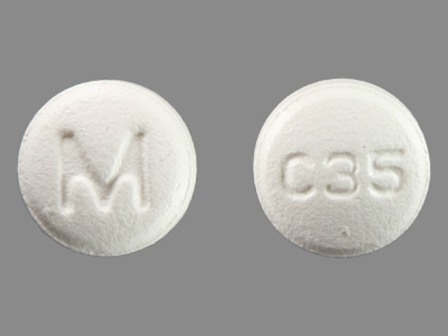
Cetirizine Hydrochloride 5 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0378-3637 Dec 14, 2009
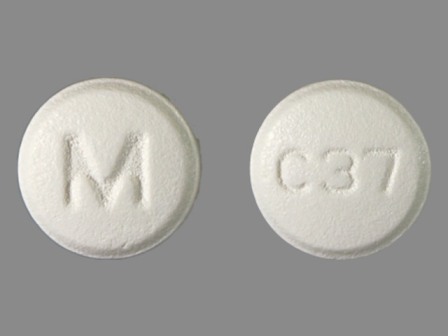
Cetirizine Hydrochloride 10 mg Oral Tablet by Mylan Pharmaceuticals Inc.
0536-1041 Dec 27, 2007
Cetirizine Hydrochloride 10 mg Oral Tablet, Film Coated by Rugby Laboratories
0904-5852 Jun 22, 2016
Cetirizine Hydrochloride 10 mg Oral Tablet by Major Pharmaceuticals
16571-402 Oct 01, 2009
Cetirizine Hydrochloride 10 mg Oral Tablet by Pack Pharmaceuticals LLC
16714-271 Oct 19, 2009
Cetirizine Hydrochloride 10 mg Oral Tablet by Northstar Rx LLC
45802-919 Dec 27, 2007
Cetirizine Hydrochloride 10 mg Oral Tablet by Perrigo New York Inc
60505-2633 Dec 27, 2007
Cetirizine Hydrochloride 10 mg Oral Tablet by Apotex Corp.
0179-8301 Dec 13, 2013
Cetirizine Hydrochloride 10 mg/1 Oral Tablet by Kaiser Foundation Hospitals
0615-8240 Dec 27, 2007
Cetirizine Hydrochloride 10 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
10544-670 Oct 01, 2009
Cetirizine Hydrochloride 10 mg Oral Tablet by Blenheim Pharmacal, Inc.
11822-6053 May 25, 2017
Cetirizine Hydrochloride 10 mg Oral Tablet, Film Coated by Rite Aid Corporation
11822-6059 May 25, 2017
Cetirizine Hydrochloride 10 mg Oral Tablet, Film Coated by Rite Aid Corporation
11822-7053 May 25, 2017
Cetirizine Hydrochloride 10 mg Oral Tablet, Film Coated by Rite Aid Corporation
33261-393 Oct 01, 2009
Cetirizine Hydrochloride 10 mg Oral Tablet by Aidarex Pharmaceuticals LLC
35356-085 Feb 23, 2012
Cetirizine Hydrochloride 10 mg Oral Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
35356-864 Oct 01, 2009
Cetirizine Hydrochloride 10 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
49348-939 Aug 01, 2011
Cetirizine Hydrochloride 10 mg Oral Tablet by Mckesson
49349-373 Mar 26, 2015
Cetirizine Hydrochloride 10 mg Oral Tablet by Remedyrepack Inc.
50090-1089 Oct 01, 2009
Cetirizine Hydrochloride 10 mg Oral Tablet by A-s Medication Solutions
Page: 1 Start a Discussion
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .