Carbamazepine - NDC Database (Page 2)
281 records found
Start a Discussion
51672-4124 Mar 31, 2009
Carbamazepine 200 mg 12 Hr Extended Release Tablet by Taro Pharmaceuticals U.S.a., Inc.
51672-4125 Mar 31, 2009
Carbamazepine 400 mg 12 Hr Extended Release Tablet by Taro Pharmaceuticals U.S.a., Inc.
60429-032 Mar 07, 2014
Carbamazepine 200 mg Oral Tablet by Golden State Medical Supply, Inc.
60429-932 Jul 20, 2011
Carbamazepine 200 mg Oral Tablet by Golden State Medical Supply, Inc.
60429-934 Jul 20, 2011
Carbamazepine 100 mg Chewable Tablet by Golden State Medical Supply, Inc.
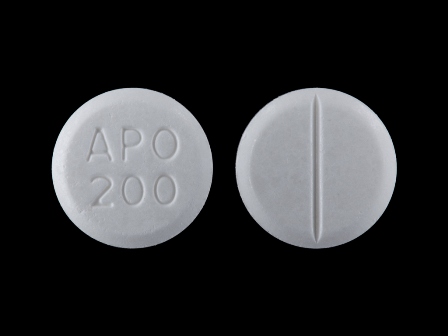
60505-0183 Sep 01, 2002
Carbamazepine 200 mg Oral Tablet by Apotex Corp.
60687-311 Aug 01, 2019
Carbamazepine 200 mg Oral Tablet, Extended Release by American Health Packaging
60687-335 Aug 01, 2019
Carbamazepine 400 mg Oral Tablet, Extended Release by American Health Packaging
60687-479 Jan 03, 2020
Carbamazepine 100 mg Oral Tablet, Chewable by American Health Packaging
60687-490 Jan 03, 2020
Carbamazepine 200 mg Oral Tablet by American Health Packaging
66993-408 Dec 15, 2011
Carbamazepine 200 mg 12 Hr Extended Release Capsule by Prasco Laboratories
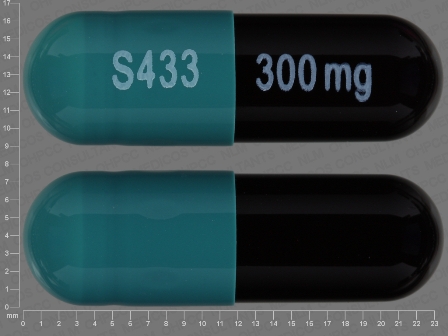
66993-409 Dec 15, 2011
Carbamazepine 300 mg 12 Hr Extended Release Capsule by Prasco Laboratories
68084-444 Jun 02, 2023
Carbamazepine 200 mg Oral Tablet by American Health Packaging
68084-561 Jun 24, 2013
Carbamazepine 200 mg 12 Hr Extended Release Tablet by American Health Packaging
68084-562 Jun 24, 2013
Carbamazepine 400 mg 12 Hr Extended Release Tablet by American Health Packaging
0179-0026 Oct 28, 2009
Carbamazepine 200 mg 12 Hr Extended Release Tablet by Kaiser Foundation Hospitals
0615-3505 Sep 30, 1990
Carbamazepine 200 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
0904-6172 Sep 20, 2010
Carbamazepine 200 mg Oral Tablet by Major Pharmaceuticals
21695-724 Jul 01, 2009
Carbamazepine 200 mg Oral Tablet by Rebel Distributors Corp
24236-024 May 12, 2008
Carbamazepine 200 mg Oral Tablet by Remedyrepack Inc.
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us .