Budesonide - NDC Database

Budesonide 3 mg 24 Hr Extended Release Enteric Coated Capsule by Mylan Pharmaceuticals Inc.
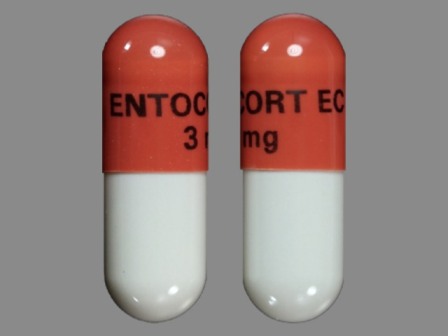
Budesonide 3 mg 24 Hr Extended Release Enteric Coated Capsule by Par Pharmaceutical Inc.

Budesonide 3 mg 24 Hr Extended Release Enteric Coated Capsule by Udl Laboratories, Inc.
0093-6815 Jan 11, 2019
Budesonide .25 mg/2ml Respiratory (Inhalation) Suspension by Teva Pharmaceuticals USA Inc
Budesonide .25 mg/2ml Respiratory (Inhalation) Suspension by Teva Pharmaceuticals USA Inc
0093-6816 Jan 11, 2019
Budesonide .5 mg/2ml Respiratory (Inhalation) Suspension by Teva Pharmaceuticals USA Inc
Budesonide .5 mg/2ml Respiratory (Inhalation) Suspension by Teva Pharmaceuticals USA Inc
0093-6817 Mar 11, 2016
Budesonide 1 mg/2ml Respiratory (Inhalation) Suspension by Teva Pharmaceuticals USA Inc
Budesonide 1 mg/2ml Respiratory (Inhalation) Suspension by Teva Pharmaceuticals USA Inc
0093-7445 Jun 23, 2016
Budesonide (Enteric Coated) 3 mg Oral Capsule, Delayed Release Pellets by Teva Pharmaceuticals USA, Inc.
Budesonide (Enteric Coated) 3 mg Oral Capsule, Delayed Release Pellets by Teva Pharmaceuticals USA, Inc.
0115-1687 Aug 04, 2016
Budesonide Inhalation Inhalation .25 mg/2ml Respiratory (Inhalation) Suspension by Impax Generics
Budesonide Inhalation Inhalation .25 mg/2ml Respiratory (Inhalation) Suspension by Impax Generics
0115-1689 Aug 04, 2016
Budesonide Inhalation Inhalation .5 mg/2ml Respiratory (Inhalation) Suspension by Impax Generics
Budesonide Inhalation Inhalation .5 mg/2ml Respiratory (Inhalation) Suspension by Impax Generics
0186-0916 Mar 19, 2007
Pulmicort 180 ug/1 Respiratory (Inhalation) Aerosol, Powder by Astrazeneca Lp
Pulmicort 180 ug/1 Respiratory (Inhalation) Aerosol, Powder by Astrazeneca Lp
0186-1988 Sep 08, 2000
Pulmicort Respules .25 mg/2ml Respiratory (Inhalation) Suspension by Astrazeneca Lp
Pulmicort Respules .25 mg/2ml Respiratory (Inhalation) Suspension by Astrazeneca Lp
0186-1989 Sep 08, 2000
Pulmicort Respules .5 mg/2ml Respiratory (Inhalation) Suspension by Astrazeneca Lp
Pulmicort Respules .5 mg/2ml Respiratory (Inhalation) Suspension by Astrazeneca Lp
0186-1990 Sep 17, 2007
Pulmicort Respules 1 mg/2ml Respiratory (Inhalation) Suspension by Astrazeneca Lp
Pulmicort Respules 1 mg/2ml Respiratory (Inhalation) Suspension by Astrazeneca Lp
0378-4500 Oct 20, 2020
Budesonide 9 mg Oral Tablet, Film Coated, Extended Release by Mylan Pharmaceuticals Inc.
Budesonide 9 mg Oral Tablet, Film Coated, Extended Release by Mylan Pharmaceuticals Inc.
0487-9601 Oct 01, 2013
Budesonide .25 mg/2ml Respiratory (Inhalation) Inhalant by Nephron Pharmaceuticals Corporation
Budesonide .25 mg/2ml Respiratory (Inhalation) Inhalant by Nephron Pharmaceuticals Corporation
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.