Amiodarone - NDC Database (Page 2)

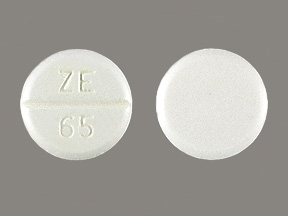
Amiodarone Hydrochloride 200 mg Oral Tablet by Golden State Medical Supply, Inc.

Amiodarone Hydrochloride 200 mg Oral Tablet by Mckesson Packaging Services a Business Unit of Mckesson Corporation
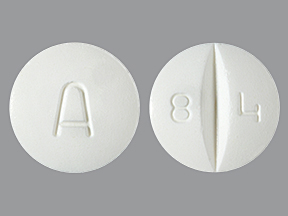
Amiodarone Hydrochloride 200 mg Oral Tablet by Denton Pharma, Inc. Dba Northwind Pharmaceuticals
0143-9875 Feb 25, 2008
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by West-ward Pharmaceutical Corp
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by West-ward Pharmaceutical Corp
0404-9772 Oct 29, 2025
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Henry Schein, Inc.
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Henry Schein, Inc.
0404-9815 Jan 08, 2022
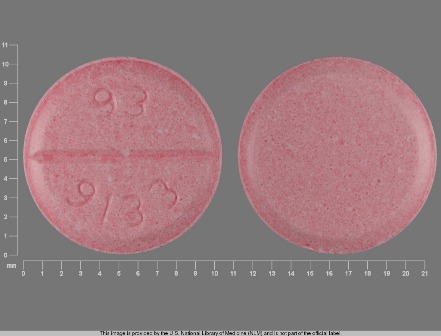
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Henry Schein, Inc
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Henry Schein, Inc
0409-1933 Nov 25, 2013
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Hospira, Inc.
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Hospira, Inc.
0409-1934 Nov 25, 2013
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Hospira, Inc.
Amiodarone Hydrochloride 50 mg/ml Intravenous Injection, Solution by Hospira, Inc.
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.