70518-1881 : Tamoxifen Citrate 20 mg Oral Tablet
| NDC: | 70518-1881 |
| Labeler: | Remedyrepack Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: | Tamoxifen Citrate |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA070929 |
| Rev. Date: |
Appearance:
| Markings: | 2233;WPI |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 10 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
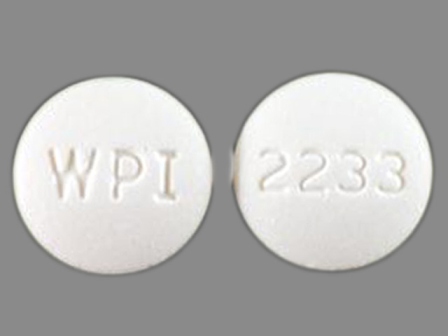
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 70518-1881-0: 30 POUCH IN 1 BOX, UNIT‑DOSE (70518‑1881‑0) > 1 TABLET IN 1 POUCH (70518‑1881‑1)
Active Ingredients:
- Tamoxifen Citrate
Dosage Strength:
- 20 mg
Inactive Ingredients:
- Croscarmellose Sodium
- Lactose Monohydrate
- Magnesium Stearate
- Cellulose, Microcrystalline
- Starch, Corn /
Pharmaceutical Classes:
- Estrogen Agonist/Antagonist [EPC]
- Selective Estrogen Receptor Modulators [MoA]
Related Products:
Based on records with the same trade name.- 70518-2721 Tamoxifen Citrate 20 mg Oral Tablet by Remedyrepack Inc.
- 0093-0782 Tamoxifen 20 mg (Tamoxifen Citrate 30.4 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-0784 Tamoxifen 10 mg (Tamoxifen Citrate 15.2 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0179-0224 Tamoxifen Citrate 10 mg Oral Tablet, Film Coated by Kaiser Foundation Hospitals
- 0179-1952 Tamoxifen Citrate 10 mg Oral Tablet, Film Coated by Kaiser Foundation Hospitals
- 0378-0144 Tamoxifen 10 mg (Tamoxifen Citrate 15.2 mg) Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0274 Tamoxifen 20 mg (Tamoxifen Citrate 30.4 mg) Oral Tablet by Mylan Pharmaceuticals Inc.
- 0591-2232 Tamoxifen 10 mg (Tamoxifen Citrate 15.2 mg) Oral Tablet by Watson Laboratories, Inc.
- 0591-2233 Tamoxifen 20 mg (Tamoxifen Citrate 30.4 mg) Oral Tablet by Watson Laboratories, Inc.
- 0591-2472 Tamoxifen 10 mg (Tamoxifen Citrate 15.2 mg) Oral Tablet by Watson Laboratories, Inc.
- 0591-2473 Tamoxifen 20 mg (Tamoxifen Citrate 30.4 mg) Oral Tablet by Watson Laboratories, Inc.
- 50090-0485 Tamoxifen Citrate 10 mg Oral Tablet by A-s Medication Solutions
- 50090-0942 Tamoxifen Citrate 20 mg Oral Tablet by A-s Medication Solutions
- 50090-1998 Tamoxifen Citrate 10 mg Oral Tablet, Film Coated by A-s Medication Solutions
- 50090-2533 Tamoxifen Citrate 10 mg Oral Tablet by A-s Medication Solutions
- 51407-439 Tamoxifen Citrate 10 mg Oral Tablet, Film Coated by Golden State Medical Supply, Inc.
- 51407-440 Tamoxifen Citrate 20 mg Oral Tablet, Film Coated by Golden State Medical Supply, Inc.
- 51862-446 Tamoxifen Citrate 20 mg Oral Tablet, Film Coated by Mayne Pharma Inc.
- 51862-447 Tamoxifen Citrate 10 mg Oral Tablet, Film Coated by Mayne Pharma Inc.
- 51862-449 Tamoxifen Citrate 10 mg Oral Tablet by Mayne Pharma Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 70518-1880Next: 70518-1882 >
Related Discussions:
Tamoxifen and breast cancer
Fellow breast cancer survivors beware of Tamoxifen! I have just found out that Tamoxifen Increases your chances of getti... 15 replies
Fellow breast cancer survivors beware of Tamoxifen! I have just found out that Tamoxifen Increases your chances of getti... 15 replies
Tamoxifen question
Has anyone experienced severe muscle spasms especially around the body beneath the breast. I have been on tamoxifen for ... 9 replies
Has anyone experienced severe muscle spasms especially around the body beneath the breast. I have been on tamoxifen for ... 9 replies
Tamoxifen / Numbness in hands and feet
I have been on Tamoxifen for almost five years and have had many of the side effects - horrendous hot flashes, joint pai... 5 replies
I have been on Tamoxifen for almost five years and have had many of the side effects - horrendous hot flashes, joint pai... 5 replies
Tamoxifen cured my migraines
I just started Tamoxifen 10 days ago after lumpectomy and radiation for ER+. Breast cancer. Clear margins, sentinel node... 3 replies
I just started Tamoxifen 10 days ago after lumpectomy and radiation for ER+. Breast cancer. Clear margins, sentinel node... 3 replies
Tamoxifen & Hot Flushes
I am 51 and it is now a year since I had a lumpectomy. Prior to starting taking Tamoxifen I was having regular periods b... 2 replies
I am 51 and it is now a year since I had a lumpectomy. Prior to starting taking Tamoxifen I was having regular periods b... 2 replies
Tamoxifen too
I did five years of this as I was advised that this would help. I think I had more things wrong with me in that five yea... 2 replies
I did five years of this as I was advised that this would help. I think I had more things wrong with me in that five yea... 2 replies
Tamoxifen and well woman Dr visit
I want to ask women on Tamoxifen, will your visit to OB-GYN be considered preventive care, or will any part of it be bil... 1 reply
I want to ask women on Tamoxifen, will your visit to OB-GYN be considered preventive care, or will any part of it be bil... 1 reply
Tamoxifen from 5 to 10 years.
I have been on tamoxifen for over 5 years. I was excited to go off of it from all the side effects. She now told me the ... 1 reply
I have been on tamoxifen for over 5 years. I was excited to go off of it from all the side effects. She now told me the ... 1 reply
Tamoxifen Ewfill
I can not refill for two days. Will I be ok ## Hello, Amanda! How are you? I'd suggest checking with your doctor. Wh... 1 reply
I can not refill for two days. Will I be ok ## Hello, Amanda! How are you? I'd suggest checking with your doctor. Wh... 1 reply
Tamoxifen Weight Gain
Experienced weight gain while taking tamoxifen and effexor will I lose when I get off these medicinesB ## Yes, there is ... 1 reply
Experienced weight gain while taking tamoxifen and effexor will I lose when I get off these medicinesB ## Yes, there is ... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
