68084-917 : Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release
| NDC: | 68084-917 |
| Labeler: | American Health Packaging |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Propafenone Hydrochloride Propafenone Hydrochloride |
| Dosage Form: | Oral Capsule, Extended Release |
| Application #: | ANDA078540 |
| Rev. Date: |
Appearance:
| Markings: | par;210 |
| Shapes: |
Capsule |
| Colors: |
 Orange Orange |
| Size (mm): | 23 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
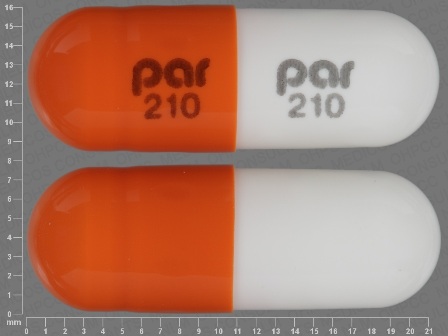
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 68084-917-32: 20 BLISTER PACK IN 1 BOX, UNIT‑DOSE (68084‑917‑32) > 1 CAPSULE, EXTENDED RELEASE IN 1 BLISTER PACK (68084‑917‑33)
Active Ingredients:
- Propafenone Hydrochloride
Dosage Strength:
- 325 mg
Inactive Ingredients:
- Anhydrous Lactose
- Magnesium Stearate
- Povidones
- Fd&c Blue No. 1
- Gelatin
- Titanium Dioxide
- D&c Yellow No. 10
- Ferrosoferric Oxide
- Butyl Alcohol
- Propylene Glycol
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Shellac
- Alcohol
- Ethylcelluloses
Pharmaceutical Classes:
- Antiarrhythmic [EPC]
Related Products:
Based on records with the same trade name.- 68084-858 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by American Health Packaging
- 60687-185 Propafenone Hydrochloride 225 1/1 Oral Capsule, Extended Release by American Health Packaging
- 60687-537 Propafenone Hydrochloride 150 mg Oral Tablet, Film Coated by American Health Packaging
- 60687-674 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by American Health Packaging
- 60687-685 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by American Health Packaging
- 60687-709 Propafenone Hydrochloride 150 mg Oral Tablet, Film Coated by American Health Packaging
- 0378-1258 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-1259 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-1261 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0591-2285 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0591-2286 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0591-2287 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0603-5448 Propafenone Hydrochloride 150 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5449 Propafenone Hydrochloride 225 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5450 Propafenone Hydrochloride 300 mg Oral Tablet by Qualitest Pharmaceuticals
- 0615-7574 Propafenone Hydrochloride 150 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0832-0740 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Upsher-smith Laboratories, LLC
- 0832-0741 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Upsher-smith Laboratories, LLC
- 0832-0742 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Upsher-smith Laboratories, LLC
- 16571-736 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Rising Pharma Holdings, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 68084-916Next: 68084-918 >
Related Discussions:
Propafenone HCl
## Has anyone else experienced a trembling/vibrating/buzzing sensation after taking this drug? If so, did you ever find ... 1 reply
## Has anyone else experienced a trembling/vibrating/buzzing sensation after taking this drug? If so, did you ever find ... 1 reply
Propafenone+V5124
Different code than previous rx...
Different code than previous rx...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.