0615-7574 : Propafenone Hydrochloride 150 mg Oral Tablet
| NDC: | 0615-7574 |
| Labeler: | Ncs Healthcare of Ky, Inc Dba Vangard Labs |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Propafenone Hydrochloride Propafenone Hydrochloride |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | ANDA075938 |
| Rev. Date: |
Appearance:
| Markings: | 5124;V |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 9 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
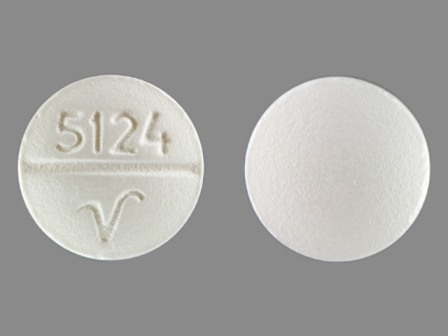
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0615-7574-39: 30 TABLET, FILM COATED IN 1 BLISTER PACK (0615‑7574‑39)
Active Ingredients:
- Propafenone Hydrochloride
Dosage Strength:
- 150 mg
Inactive Ingredients:
- Carnauba Wax
- Hypromellose
- Lactose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Povidone
- Starch, Corn
- Sodium Starch Glycolate Type a Potato
- Stearic Acid
- Titanium Dioxide
- Triacetin
Pharmaceutical Classes:
- Antiarrhythmic [EPC]
Related Products:
Based on records with the same trade name.- 0378-1258 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-1259 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-1261 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0591-2285 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0591-2286 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0591-2287 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0603-5448 Propafenone Hydrochloride 150 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5449 Propafenone Hydrochloride 225 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5450 Propafenone Hydrochloride 300 mg Oral Tablet by Qualitest Pharmaceuticals
- 0832-0740 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Upsher-smith Laboratories, LLC
- 0832-0741 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Upsher-smith Laboratories, LLC
- 0832-0742 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Upsher-smith Laboratories, LLC
- 16571-736 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Rising Pharma Holdings, Inc.
- 16571-737 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Rising Pharma Holdings, Inc.
- 16571-738 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Rising Pharma Holdings, Inc.
- 16714-825 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Northstar Rx LLC
- 16714-826 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Northstar Rx LLC
- 16714-827 Propafenone Hydrochloride 425 mg Oral Capsule, Extended Release by Northstar Rx LLC
- 24979-085 Propafenone Hydrochloride 225 mg Oral Capsule, Extended Release by Twi Pharmaceuticals, Inc.
- 24979-086 Propafenone Hydrochloride 325 mg Oral Capsule, Extended Release by Twi Pharmaceuticals, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0615-7573Next: 0615-7575 >
Related Discussions:
Propafenone HCl
## Has anyone else experienced a trembling/vibrating/buzzing sensation after taking this drug? If so, did you ever find ... 1 reply
## Has anyone else experienced a trembling/vibrating/buzzing sensation after taking this drug? If so, did you ever find ... 1 reply
Propafenone+V5124
Different code than previous rx...
Different code than previous rx...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.