54868-5388 : Dextroamphetamine Sulfate 15 mg Extended Release Capsule
| NDC: | 54868-5388 |
| Labeler: | Physicians Total Care, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Dextroamphetamine Sulfate Dextroamphetamine Sulfate |
| Dosage Form: | Oral Capsule, Extended Release |
| Application #: | ANDA076137 |
| Rev. Date: | |
| CSA Schedule: | CII (US) [1] |
[1] Schedule II / IIN Controlled Substance: High potential for abuse which may lead to severe psychological or physical dependence. (i.e. Narcotics such as Dilaudid, Methadone, Demerol, Oxycodone, Percocet, Fentanyl, Morphine, Opium, Codeine, and Hydrocodone ... Schedule IIN stimulants include non-narcotic Amphetamines such as Dexedrine, Adderall, Desoxyn, Methylphenidate (Ritalin) ... Other Schedule II substances include Amobarbital, Glutethimide, and Pentobarbital. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | barr;956 |
| Shapes: |
Capsule |
| Colors: |
 Brown Brown |
| Size (mm): | 16 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
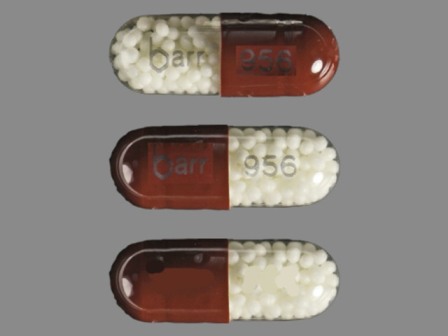
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54868-5388-0: 10 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE, PLASTIC (54868‑5388‑0)
- 54868-5388-1: 30 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE, PLASTIC (54868‑5388‑1)
Active Ingredients:
- Dextroamphetamine Sulfate
Dosage Strength:
- 15 mg
Inactive Ingredients:
- Silicon Dioxide
- Dibutyl Sebacate
- Ethylcelluloses
- Methylcellulose (15 Cps)
- Povidone
- Propylene Glycol
- Sucrose
- Starch, Corn
- Talc
- Ferrosoferric Oxide
- Gelatin
- Ferric Oxide Red
- Titanium Dioxide
- Ferric Oxide Yellow
- D&c Yellow No. 10
- Fd&c Blue No. 1
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Aluminum Oxide
- Shellac
Pharmaceutical Classes:
- Central Nervous System Stimulant [EPC]
- Central Nervous System Stimulation [PE]
Related Products:
Based on records with the same trade name.- 54868-5479 Dextroamphetamine Sulfate 10 mg Extended Release Capsule by Physicians Total Care, Inc.
- 0378-1070 Dextroamphetamine Sulfate 5 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-1071 Dextroamphetamine Sulfate 10 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-1072 Dextroamphetamine Sulfate 15 mg Oral Capsule, Extended Release by Mylan Pharmaceuticals Inc.
- 0406-8958 Dextroamphetamine Sulfate 5 mg Oral Tablet by Mallinckrodt, Inc.
- 0406-8959 Dextroamphetamine Sulfate 10 mg Oral Tablet by Mallinckrodt, Inc.
- 0406-8960 Dextroamphetamine Sulfate 5 mg Extended Release Capsule by Mallinckrodt Inc.
- 0406-8961 Dextroamphetamine Sulfate 10 mg Extended Release Capsule by Mallinckrodt Inc.
- 0406-8962 Dextroamphetamine Sulfate 15 mg Extended Release Capsule by Mallinckrodt Inc.
- 0555-0952 Dextroamphetamine Sulfate 5 mg Oral Tablet by Barr Laboratories Inc.
- 0555-0953 Dextroamphetamine Sulfate 10 mg Oral Tablet by Barr Laboratories Inc.
- 0555-0954 Dextroamphetamine Sulfate 5 mg Extended Release Capsule by Barr Laboratories Inc.
- 0555-0955 Dextroamphetamine Sulfate 10 mg Extended Release Capsule by Barr Laboratories Inc.
- 0555-0956 Dextroamphetamine Sulfate 15 mg Extended Release Capsule by Barr Laboratories Inc.
- 0603-2674 Dextroamphetamine Sulfate 5 mg Oral Capsule, Extended Release by Par Pharmaceutical
- 0603-2675 Dextroamphetamine Sulfate 10 mg Oral Capsule, Extended Release by Par Pharmaceutical
- 0603-2676 Dextroamphetamine Sulfate 15 mg Oral Capsule, Extended Release by Par Pharmaceutical
- 10702-065 Dextroamphetamine Sulfate 5 mg Oral Tablet by Kvk-tech, Inc.
- 10702-066 Dextroamphetamine Sulfate 10 mg Oral Tablet by Kvk-tech, Inc.
- 11534-188 Dextroamphetamine Sulfate 5 mg Oral Tablet by Sunrise Pharmaceutical, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54868-5387Next: 54868-5389 >
Related Discussions:
Teva Barr Dextroamphetamine sulfate fraud placebo
I have been on Barr/Teva dexedrine sulfate (generic) for 14 years. After 5 "shortages" I recently received back ... 30 replies
I have been on Barr/Teva dexedrine sulfate (generic) for 14 years. After 5 "shortages" I recently received back ... 30 replies
Winder Labs Dextroamphetamine Sulfate Quality
I've never heard of or taken Winder Labs brand and my CVS has them in stock. This is always concerning as ONLY Teva/... 14 replies
I've never heard of or taken Winder Labs brand and my CVS has them in stock. This is always concerning as ONLY Teva/... 14 replies
dextroamphetamine and medicare
My mother was told that her doctor could no longer prescribe the dextroamphetamine that she has been taking, at minimum ... 37 replies
My mother was told that her doctor could no longer prescribe the dextroamphetamine that she has been taking, at minimum ... 37 replies
Dextroamphetamine 10mg Tabs
I am trying to find a pharmacy in my area that can fill my prescription for Dextroamphetamine 10 mg tabs. All the pharma... 23 replies
I am trying to find a pharmacy in my area that can fill my prescription for Dextroamphetamine 10 mg tabs. All the pharma... 23 replies
dextroamphetamine for narcolepsy
used for 10 years for narcolepsy ## I have been useing dextroamphetamine 5mg tablets for 4 years for narcolepsy. I also ... 10 replies
used for 10 years for narcolepsy ## I have been useing dextroamphetamine 5mg tablets for 4 years for narcolepsy. I also ... 10 replies
Dextroamphetamine Price
Ive taken Dexadrine tabs for a couple years. The price is now too much for me too afford. No inssurance. My Dr. gave me ... 7 replies
Ive taken Dexadrine tabs for a couple years. The price is now too much for me too afford. No inssurance. My Dr. gave me ... 7 replies
dextroamphetamine instant release core 10mg
Has Core Pharmaceuticals improved their product since the year 2009? When I last tried the Core 10mg generic version of ... 6 replies
Has Core Pharmaceuticals improved their product since the year 2009? When I last tried the Core 10mg generic version of ... 6 replies
Dextroamphetamine ER spansules generic experiences?
Hey all, I take Dex IR for ADHD-C in the afternoons and it works great. I need to find an XR medication to take in the A... 5 replies
Hey all, I take Dex IR for ADHD-C in the afternoons and it works great. I need to find an XR medication to take in the A... 5 replies
dextroamphetamine pictures
ROUND PINK 953/10 B ## picturea ## I cannot post a picture, due to copyright reasons, however, I can tell you from the i... 4 replies
ROUND PINK 953/10 B ## picturea ## I cannot post a picture, due to copyright reasons, however, I can tell you from the i... 4 replies
DEXTROAMPHETAMINE 30 MG
MY DOCTOR SUDDENLY PASSED AWAY DUE TO HEART ATTACK IM TRYING TO FIND A DOCTOR WHO WILL PRESCIBE THIS FOR ME.I LIVE IN MU... 3 replies
MY DOCTOR SUDDENLY PASSED AWAY DUE TO HEART ATTACK IM TRYING TO FIND A DOCTOR WHO WILL PRESCIBE THIS FOR ME.I LIVE IN MU... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.