54868-0092 : Flurazepam Hydrochloride 15 mg Oral Capsule
| NDC: | 54868-0092 |
| Labeler: | Physicians Total Care, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Flurazepam Hydrochloride Flurazepam Hydrochloride |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA070345 |
| Rev. Date: | |
| CSA Schedule: | CIV (US) [1] |
[1] Schedule IV Controlled Substance: Low potential for abuse relative to substances in Schedule III. Examples include Alprazolam (Xanax), Diazepam (Valium), Carisoprodol (Soma), Clonazepam (Klonopin), Lorazepam (Ativan), Clorazepate (Tranxene), Midazolam (Versed), Temazepam (Restoril), and Triazolam (Halcion).. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | MYLAN;4415 |
| Shapes: |
Capsule |
| Colors: |
 White / White /
 Blue Blue |
| Size (mm): | 15 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
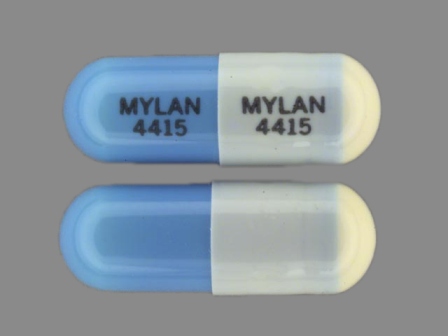
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54868-0092-1: 30 CAPSULE IN 1 BOTTLE, PLASTIC (54868‑0092‑1)
- 54868-0092-2: 20 CAPSULE IN 1 BOTTLE, PLASTIC (54868‑0092‑2)
Active Ingredients:
- Flurazepam Hydrochloride
Dosage Strength:
- 15 mg
Inactive Ingredients:
- Silicon Dioxide
- Magnesium Stearate
- Cellulose, Microcrystalline
- Powdered Cellulose
- Sodium Lauryl Sulfate
- Fd&c Blue No. 1
- Fd&c Red No. 3
- Gelatin
- Titanium Dioxide
- Ferrosoferric Oxide
- D&c Yellow No. 10
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Propylene Glycol
- Shellac
Pharmaceutical Classes:
- Benzodiazepine [EPC]
- Benzodiazepines [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 54868-0093 Flurazepam Hydrochloride 30 mg Oral Capsule by Physicians Total Care, Inc.
- 0378-4415 Flurazepam Hydrochloride 15 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-4430 Flurazepam Hydrochloride 30 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 55289-038 Flurazepam Hydrochloride 30 mg Oral Capsule by Pd-rx Pharmaceuticals, Inc.
- 62135-736 Flurazepam Hydrochloride 15 mg Oral Capsule by Chartwell Rx, LLC
- 62135-737 Flurazepam Hydrochloride 30 mg Oral Capsule by Chartwell Rx, LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54868-0091Next: 54868-0093 >
Related Discussions:
flurazepam 30
blue casule ## I have a bottle of flurazepam 30 mg, I found in my drawer from 2010 when I suffered from insomnia back th... 3 replies
blue casule ## I have a bottle of flurazepam 30 mg, I found in my drawer from 2010 when I suffered from insomnia back th... 3 replies
Is Flurazepam still available?
I have been on Flurazepam for some time and am being told by my pharmacy that there is a manufacture problem and they do... 12 replies
I have been on Flurazepam for some time and am being told by my pharmacy that there is a manufacture problem and they do... 12 replies
Mylan Discontinued Flurazepam
Desperate times again. What to do? Where to go? This is absurd. Now back where we were 2 years ago, no one else makes it... 20 replies
Desperate times again. What to do? Where to go? This is absurd. Now back where we were 2 years ago, no one else makes it... 20 replies
Chartwell Flurazepam
Is anyone retaking Chartwell Flurazepam and getting the same results as they had with Mylan? It did not work for me. I&#...
Is anyone retaking Chartwell Flurazepam and getting the same results as they had with Mylan? It did not work for me. I&#...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.