0113-1612 : Good Sense Allergy Relief 10 mg Oral Tablet
| NDC: | 0113-1612 |
| Labeler: | L. Perrigo Company |
| Product Type: | Human OTC Drug |
| Drug Name: |  Good Sense Allergy Relief Good Sense Allergy Relief |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA076301 |
| Rev. Date: |
Appearance:
| Markings: | L612 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 8 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
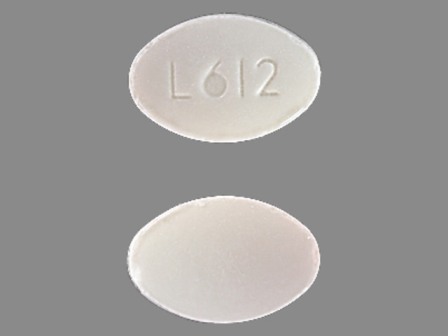
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0113-1612-95: 1 BOTTLE IN 1 CARTON (0113‑1612‑95) > 45 TABLET IN 1 BOTTLE
Active Ingredients:
- Loratadine
Dosage Strength:
- 10 mg
Inactive Ingredients:
- Lactose Monohydrate
- Magnesium Stearate
- Povidones /
Related Products:
Based on records with the same trade name.- 0113-0042 Good Sense Allergy Relief 4 mg Oral Tablet by L. Perrigo Company
- 0113-0404 Chlorpheniramine Maleate 4 mg Oral Tablet by L. Perrigo Company
- 0113-0479 Diphenhydramine Hydrochloride 25 mg Oral Tablet by L. Perrigo Company
- 0113-0612 Loratadine 10 mg 24 Hr Oral Tablet by L. Perrigo Company
- 0113-1191 Good Sense Allergy Relief 10 mg Oral Tablet, Orally Disintegrating by L. Perrigo Company
- 0113-9755 Good Sense Allergy Relief 10 mg Oral Tablet, Orally Disintegrating by L. Perrigo Company
- 0113-0462 Diphenhydramine Hydrochloride 25 mg Oral Capsule by L Perrigo Company
- 50090-7048 Good Sense Allergy Relief 10 mg Oral Tablet by A-s Medication Solutions
- 50090-7049 Good Sense Allergy Relief 10 mg Oral Tablet by A-s Medication Solutions
- 50090-7051 Good Sense Allergy Relief 10 mg Oral Tablet by A-s Medication Solutions
- 63187-167 Good Sense Allergy Relief 25 mg Oral Capsule by Proficient Rx Lp
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0113-1461Next: 0113-1619 >
Related Discussions:
Loratadine D24 Hr Tab M
how long do the side effects last? I took it last night? ## No one can really tell you how long you'll experience th... 2 replies
how long do the side effects last? I took it last night? ## No one can really tell you how long you'll experience th... 2 replies
Loratadine 10mg - OTC availability
Can Loratadine 10mg antihistamine 24-hour relief tablets (made by Perrigo) be purchased over-the-counter or does it requ... 1 reply
Can Loratadine 10mg antihistamine 24-hour relief tablets (made by Perrigo) be purchased over-the-counter or does it requ... 1 reply
IC Loratadine 10 mg
what does theas do and what is it for ## Loratidine is an antihistamine, it used to help treat allergy symptoms. Common ... 2 replies
what does theas do and what is it for ## Loratidine is an antihistamine, it used to help treat allergy symptoms. Common ... 2 replies
Taking Loratadine after heart surgery
I had Aortic Valve replacement 4 weeks ago. Now I am starting to have my usual fall allergies and starting to sneeze and... 1 reply
I had Aortic Valve replacement 4 weeks ago. Now I am starting to have my usual fall allergies and starting to sneeze and... 1 reply
claritin 10mg loratadine 428
I wanted to find out if this has Sudafed in it and if it has to have a prescription to get it? ## Hi famous, How are you... 1 reply
I wanted to find out if this has Sudafed in it and if it has to have a prescription to get it? ## Hi famous, How are you... 1 reply
Is the zylohist loratadine has steroids
the content of zylohist loratadine ## No, the active ingredient Loratidine is not a steroid, it is an antihistamine used... 3 replies
the content of zylohist loratadine ## No, the active ingredient Loratidine is not a steroid, it is an antihistamine used... 3 replies
macrodantin and loratadine histamine
I have just been prescribed Macrodantin for a UTI, 5 hours after taking this, I have shortness of breath a little bit of...
I have just been prescribed Macrodantin for a UTI, 5 hours after taking this, I have shortness of breath a little bit of...
Excessive eating or weight gain with LORATADINE, anyone??
Just recently I complain to my doctor about my allergies, he put me on loratadine 10 mg once a day. It has been brought ... 1 reply
Just recently I complain to my doctor about my allergies, he put me on loratadine 10 mg once a day. It has been brought ... 1 reply
Loratadine 10.5mg
is there any asprin an if so how much ## There is no 10.5mg Loratadine and no, that is the active ingredient, it is a ge... 1 reply
is there any asprin an if so how much ## There is no 10.5mg Loratadine and no, that is the active ingredient, it is a ge... 1 reply
LORATADINE 5MG/5 ML SYRUP TAR
CAN YOU TAKE MOTRIN WITH THIS? ## I WANT TO MAKE LORATDINE CLEAR SYRUP HOW TO MAKE IT SUCH AS FORMULA , METHOD PACKING D... 1 reply
CAN YOU TAKE MOTRIN WITH THIS? ## I WANT TO MAKE LORATDINE CLEAR SYRUP HOW TO MAKE IT SUCH AS FORMULA , METHOD PACKING D... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.