Rivastigmine
FDA Approved: * January 8, 2008Pharm Company: * WATSON LABS
Category: Alzheimers / Dementia
Rivastigmine (sold under the trade name Exelon among others) is a cholinesterase inhibitor used for the treatment of mild to moderate Alzheimer's disease.[1] The drug can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects,[2] which typically include nausea and vomiting.[3] The drug is eliminated through the urine, and appears to have relatively few drug-drug interactions.[3] ... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List

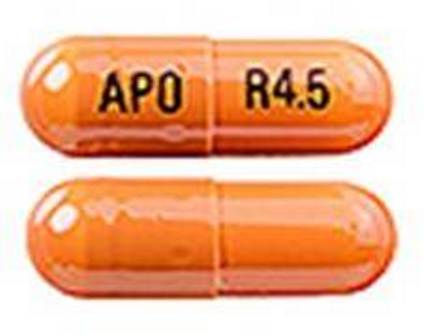
Rivastigmine 4.5 mg (As Rivastigmine Tartrate 7.2 mg) Oral Capsule
NDC: 0591-3210
Labeler:
Watson Laboratories, Inc.
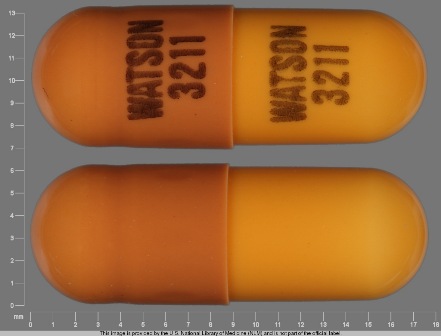
Rivastigmine 6 mg (As Rivastigmine Tartrate 9.6 mg) Oral Capsule
NDC: 0591-3211
Labeler:
Watson Laboratories, Inc.
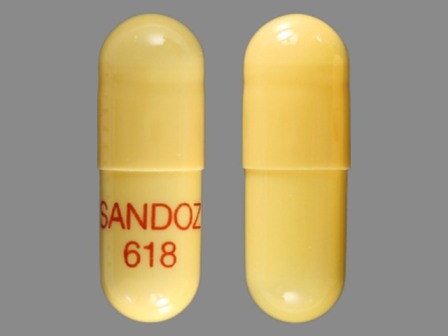
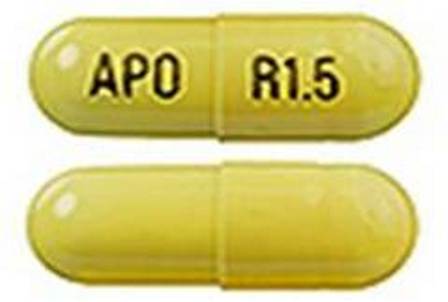
Rivastigmine 1.5 mg (As Rivastigmine Tartrate 2.4 mg) Oral Capsule
NDC: 0781-2614
Labeler:
Sandoz Inc
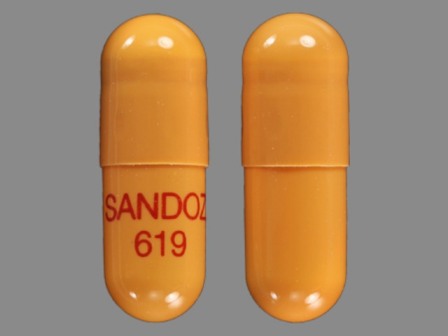

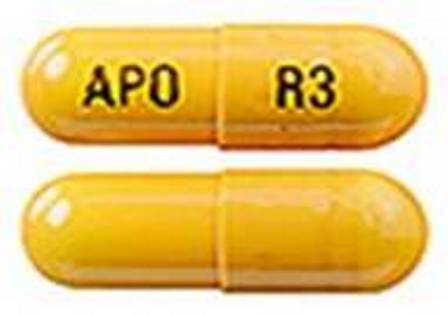
Rivastigmine 4.5 mg (As Rivastigmine Tartrate 7.2 mg) Oral Capsule
NDC: 0781-2616
Labeler:
Sandoz Inc
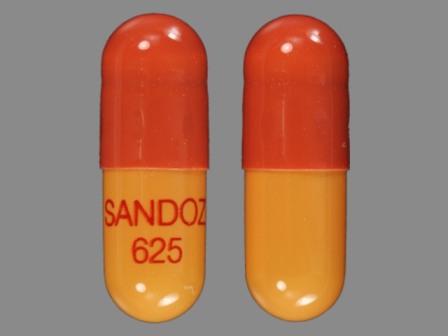
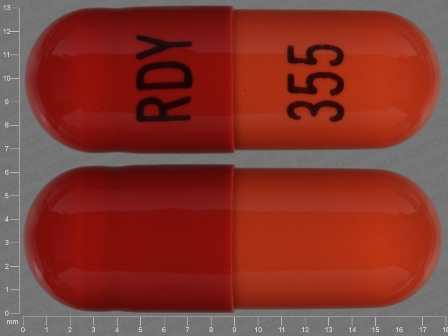
Rivastigmine 6 mg (As Rivastigmine Tartrate 9.6 mg) Oral Capsule
NDC: 55111-355
Labeler:
Dr.reddy's Laboratories Limited