Paricalcitol
FDA Approved: * September 27, 2013Pharm Company: * TEVA PHARMS
Category: Thyroid
Paricalcitol (chemically it is 19-nor-1,25-(OH)2-vitamin D2. Marketed by Abbott Laboratories under the trade name Zemplar) is a drug used for the prevention and treatment of secondary hyperparathyroidism (excessive secretion of parathyroid hormone) associated with chronic kidney failure. It is an analog of 1,25-dihydroxyergocalciferol, the active form of vitamin D2 (ergocalciferol). It was patented in 1989 and approved for medical use in 1998.[2] Contents 1 Medical uses 2 Ad... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List



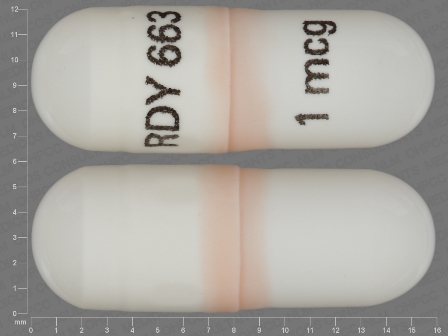
Paricalcitol 1 ug/1 Oral Capsule, Liquid Filled
NDC: 55111-663
Labeler:
Dr. Reddy's Laboratories Limited
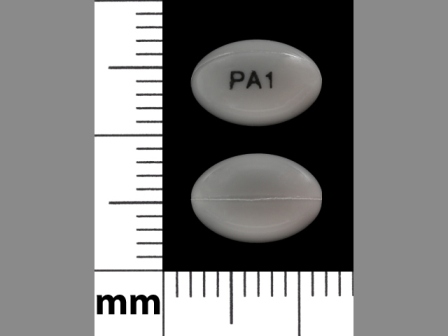
Paricalcitol 1 ug/1 Oral Capsule, Liquid Filled
NDC: 60429-078
Labeler:
Golden State Medical Supply, Inc.

Paricalcitol 2 ug/1 Oral Capsule, Liquid Filled
NDC: 60429-079
Labeler:
Golden State Medical Supply, Inc.

Paricalcitol 1 ug/1 Oral Capsule, Liquid Filled
NDC: 68382-266
Labeler:
Zydus Pharmaceuticals USA Inc

Paricalcitol 2 ug/1 Oral Capsule, Liquid Filled
NDC: 68382-267
Labeler:
Zydus Pharmaceuticals USA Inc

