Methazolamide
FDA Approved: * June 30, 1993Pharm Company: * TEVA PHARMS
Category: Vision / Eye Health
Methazolamide (trade name Neptazane) is a potent carbonic anhydrase inhibitor. It is indicated in the treatment of increased intraocular pressure (IOP) in chronic open-angle glaucoma and secondary glaucoma. Also it is used preoperatively in acute angle-closure (narrow-angle) glaucoma where lowering the IOP is desired before surgery. This drug has displayed teratogenic effects in rats. Compared to another drug in the same class, Acetazolamide, Methazolamide requires a lower dose when administ... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.3 Discussions
Dosage List




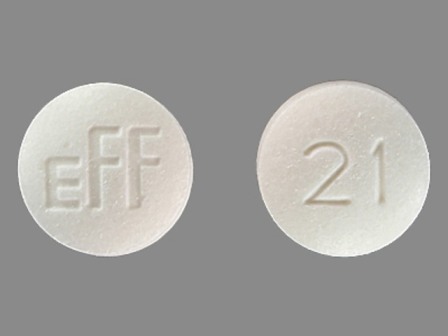
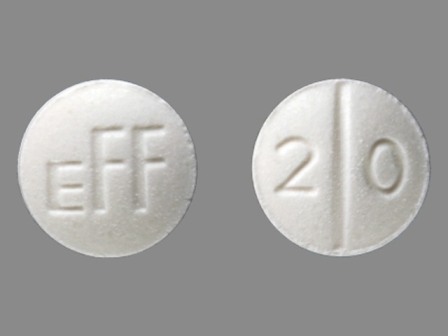
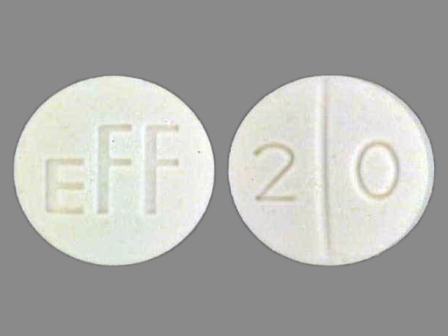



Popular Topics
Side Effects of Methazolamide 1 REPLY
1 REPLY
What are the side effects of this medication Methazolamide? ## Side effects of Methazolamide include: Dizziness, drowsin...