Lescol
Active Ingredient(s): FluvastatinFDA Approved: * December 31, 1993
Pharm Company: * NOVARTIS
Category: Cholesterol
Fluvastatin is a member of the statin drug class, used to treat hypercholesterolemia and to prevent cardiovascular disease. It was patented in 1982 and approved for medical use in 1994.[4] It is on the World Health Organization's List of Essential Medicines.[5] Contents 1 Adverse effects 2 Interactions 3 Pharmacology 3.1 Mechanism of action 3.2 Pharmacodynamics 3.3 Pharmacokinetics 4 Names 5 Research 6 References Adverse effects Adverse effects are co... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.2 Discussions
Dosage List
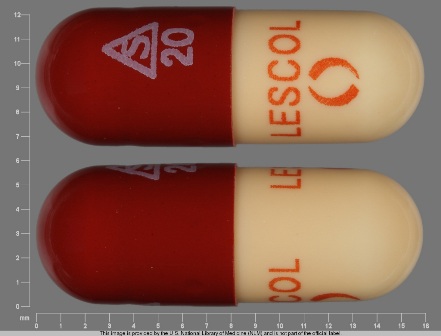
Lescol (As Fluvastatin Sodium) 20 mg Oral Capsule
NDC: 0078-0176
Labeler:
Novartis Pharmaceuticals Corporation
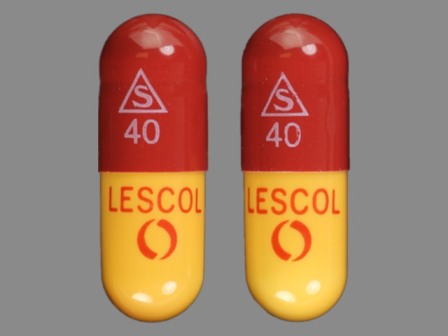
Lescol (As Fluvastatin Sodium) 40 mg Oral Capsule
NDC: 0078-0234
Labeler:
Novartis Pharmaceuticals Corporation
Lescol (As Fluvastatin Sodium) 20 mg Oral Capsule
NDC: 54868-3329
Labeler:
Physicians Total Care, Inc.
Lescol (As Fluvastatin Sodium) 40 mg Oral Capsule
NDC: 54868-4224
Labeler:
Physicians Total Care, Inc.
Related Brands
Drugs with the same active ingredientsPopular Topics
lescol xl
for cholestrol...