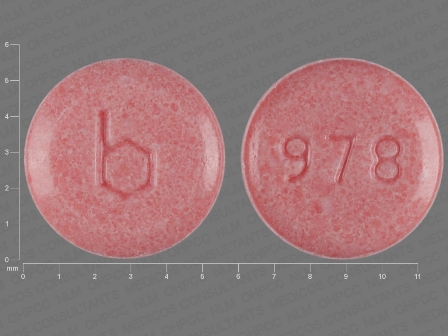
Junel 1.5/30
Active Ingredient(s): Ethinyl Estradiol + Norethindrone AcetateFDA Approved: * May 30, 2003
Pharm Company: * BARR
Category: Birth Control / Contraceptive
Ethinylestradiol/norethisterone acetate (EE/NETA), or ethinylestradiol/norethindrone acetate, is a combination of ethinylestradiol (EE) and norethisterone acetate (NETA) which is used as birth control and menopausal hormone therapy.[1][2] EE is an estrogen, while norethisterone acetate (NETA) is a progestin.[1] It is taken by mouth.[1] Some preparations of EE/NETA used in birth control additionally contain an iron supplement in t... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List

Related Brands
Drugs with the same active ingredients- Alyacen 1/35
- Alyacen 7/7/7
- Amabelz
- Aranelle
- Aurovela FE
- Balziva-21
- Balziva-28
- Balziva 35
- Blisovi 24 Fe
- Blisovi Fe 1/20
- Blisovi Fe 1.5/30
- Brevicon
- Briellyn
- Chabelina Fe
- Cyclafem 0.5/35
- Cyclafem 1/35
- Cyclafem 7/7/7
- Cyonanz
- Dasetta
- Dasetta 1/35
- Estrostep Fe
- Femhrt
- Femlyv
- Finzala
- Fyavolv
- Gemmily
- Genora 0.5/35
- Genora 1/35
- Gildagia
- Hailey Fe 1/20
- Hailey Fe 1.5/30
- Jenest-28
- Junel 1/20
- Junel 21 1/20
- Junel 21 1.5/30
- Junel Fe 1/20
- Kaitlib Fe
- Larin 1/20
- Larin 1.5/30
- Larin 24 Fe
- Larin Fe 1/20
- Larin Fe 1.5/30
- Leena
- Leribane
- Lo Loestrin Fe
- Lo Minastrin Fe
- Loestrin 21 1/20
- Loestrin 21 1.5/30
- Loestrin 24 Fe
- Loestrin Fe 1/20
- Loestrin Fe 1.5/30
- Merzee
- Mibelas 24 Fe
- Minastrin 24 Fe
- Modicon 28
- Necon 0.5/35
- Necon 1/35
- Necon 10/11
- Necon 7/7/7
- Nevis
- Nexesta Fe
- Norethin 1/35E
- Norinyl 1/35
- Nortrel 7/7/7
- Nylia 1/35
- Nylia 7/7/7
- Ortho-Novum 1/35-28
- Ortho-Novum 10/11-28
- Ortho-Novum 7/7/7-28
- Oshih
- Ovcon-35
- Ovcon 50
- Philith
- Pirmella 1/35
- Pirmella 7/7/7
- Rhuzdah
- Tri-legest 21
- Tri-legest Fe
- Tri-Norinyl
- Vyfemla
- Wera
Popular Topics
I have had to go through 7 different BC pills to find one that I did not get horrible side effects from (nortrel) now I ...
I used to take loestrin 21 1/20 and never had any issues with it. I moved and lost insurance for awhile. My new dr put m...
I am 53 and have been taking lo loestrin fe 1/10 for about two years. I stopped taking it for 2 months and have lots of ...
HELLO, MY NAME IS RICHARD, I HAVE MEDICAL AND PRESCRIPTION COVERAGE FROM THE STATE OF MD, WHERE I RETIRED. I AM IN FL AN...
Which is stronger, 30mg Oxycodone or 30mg Morphine Sulfate? ## I'm sorry that you had trouble posting, but glad you ...
round blue 224 ## I believe 224 is 30 mg immediate release Oxycodone, which is used to treat pain (moderate to severe le...
Can't find a pharmacy to fill my script for 175 oxycodone 30's. Anyone have any suggestions where to try? ## hav...
e563 on one side 30 on other ## narcotice pain medication ## I have a pill that is a small round pill and is scored with...
I was prescribed 270 30 mg oxycodone take one every 2-3 hours as needed for pain. Is this a common dose? It seems extrem...
Hello, Currently, as of March 21, 2016, I have been unable to get my 30mg Adderall generic filled after visiting over a ...