Gabitril
Active Ingredient(s): TiagabineFDA Approved: * September 30, 1997
Pharm Company: * CEPHALON
Category: Anticonvulsant
Tiagabine (trade name Gabitril) is an anticonvulsant medication produced by Cephalon that is used in the treatment of epilepsy. The drug is also used off-label in the treatment of anxiety disorders and panic disorder. Contents 1 Medical uses 2 Side effects 3 Warning 4 Overdose 5 Pharmacology 6 Pharmacodynamics 7 Monitoring Parameters 8 History 9 See also 10 References 11 External links Medical uses Tiagabine is approved by U.S. Food and Drug Administration (FDA) as an adjunctive treatm... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.6 Discussions
Dosage List
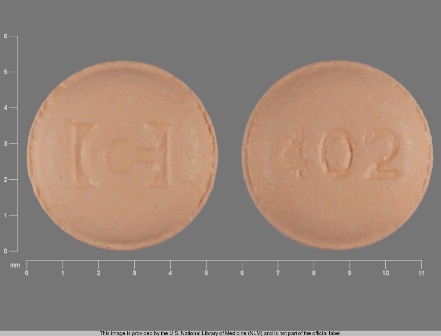





Gabitril 4 mg Oral Tablet
NDC: 49999-692
Labeler:
Lake Erie Medical & Surgial Supply Dba Quality Care Products LLC
Popular Topics
Gabitril 4mg.
1@bedtime...