Enablex
Active Ingredient(s): DarifenacinFDA Approved: * December 22, 2004
Pharm Company: * NOVARTIS
Category: Urinary System
Darifenacin (trade name Enablex in United States and Canada, Emselex in the European Union) is a medication used to treat urinary incontinence due to an overactive bladder.[1][2][3] It was discovered by scientists at the Pfizer research site in Sandwich, UK under the identifier UK-88,525 and used to be marketed by Novartis. In 2010, the US rights were sold to Warner Chilcott for US$400 million. Contents 1 Adverse effects 2 Medical uses 3 Mecha... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.8 Discussions
Dosage List
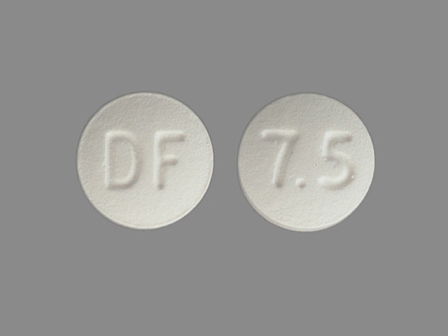
24 Hr Enablex 7.5 mg Extended Release Tablet
NDC: 0078-0419
Labeler:
Novartis Pharmaceuticals Corporation
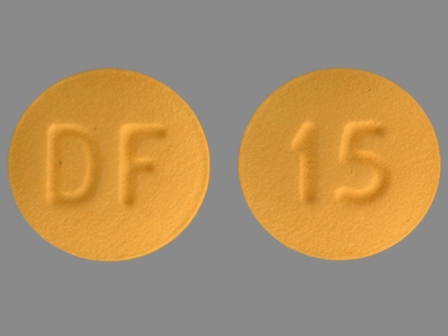
Enablex 15 mg 24 Hr Extended Release Tablet
NDC: 0078-0420
Labeler:
Novartis Pharmaceuticals Corporation
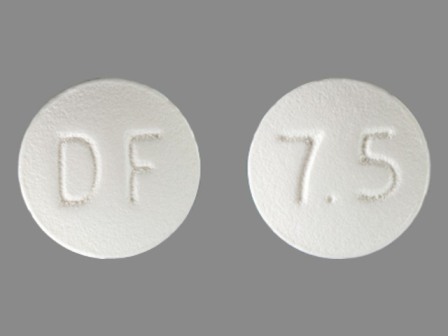
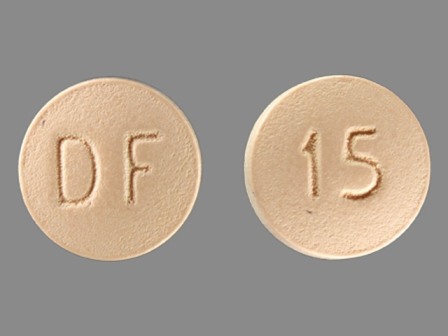

Enablex 15 mg 24 Hr Extended Release Tablet
NDC: 35356-272
Labeler:
Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC


Popular Topics
Enablex-darifenacin
Prostate Condition...
Enablex 7.5 mg.
Prescribed for bladder spammes...
enablex incontence
enablex...