69189-0549 : Rifabutin 150 mg Oral Capsule
| NDC: | 69189-0549 |
| Labeler: | Avera Mckennan Hospital |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Rifabutin Rifabutin |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA090033 |
| Rev. Date: |
Appearance:
| Markings: | LU;R01 |
| Shapes: |
Capsule |
| Colors: |
 Brown / Brown /
 Brown Brown |
| Size (mm): | 21 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
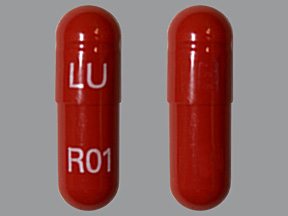
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 69189-0549-1: 1 CAPSULE IN 1 DOSE PACK (69189‑0549‑1)
Active Ingredients:
- Rifabutin
Dosage Strength:
- 150 mg
Inactive Ingredients:
- Cellulose, Microcrystalline
- Crospovidone
- Ferric Oxide Red
- Gelatin
- Magnesium Stearate
- Potassium Hydroxide
- Shellac
- Silicon Dioxide
- Sodium Lauryl Sulfate
- Titanium Dioxide
Pharmaceutical Classes:
- Rifamycin Antimycobacterial [EPC]
- Rifamycins [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 10135-738 Rifabutin 150 mg Oral Capsule by Marlex Pharmaceuticals, Inc.
- 59762-1350 Rifabutin 150 mg Oral Capsule by Greenstone LLC
- 60687-198 Rifabutin 150 mg Oral Capsule by American Health Packaging
- 68180-285 Rifabutin 150 mg Oral Capsule by Lupin Pharmaceuticals, Inc.
- 70518-4254 Rifabutin 150 mg Oral Capsule by Remedyrepack Inc.
- 70954-041 Rifabutin 150 mg Oral Capsule by Novitium Pharma LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 69189-0548Next: 69189-0551 >
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.