68788-7219 : Fluvoxamine Maleate 100 mg Oral Tablet, Coated
| NDC: | 68788-7219 |
| Labeler: | Preferred Pharmaceuticals, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Fluvoxamine Maleate Fluvoxamine Maleate |
| Dosage Form: | Oral Tablet, Coated |
| Application #: | NDA021519 |
| Rev. Date: |
Appearance:
| Markings: | 1221 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 15 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
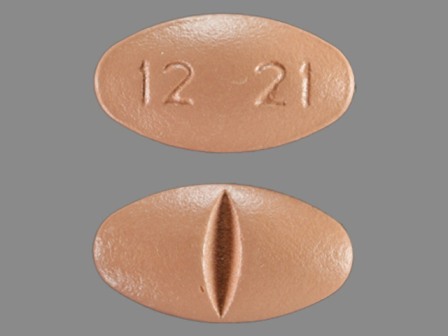
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 68788-7219-3: 30 TABLET, COATED IN 1 BOTTLE (68788‑7219‑3)
- 68788-7219-6: 60 TABLET, COATED IN 1 BOTTLE (68788‑7219‑6)
- 68788-7219-9: 90 TABLET, COATED IN 1 BOTTLE (68788‑7219‑9)
Active Ingredients:
- Fluvoxamine Maleate
Dosage Strength:
- 100 mg
Inactive Ingredients:
- Mannitol
- Carnauba Wax
- Polyethylene Glycol 400
- Polysorbate 80
- Starch, Potato
- Silicon Dioxide
- Starch, Corn
- Sodium Stearyl Fumarate
- Titanium Dioxide
- Ferric Oxide Yellow
- Ferric Oxide Red
- Hypromellose 2910 (3 Mpa.s)
- Polyethylene Glycol 8000
- Ferrosoferric Oxide
- Hypromellose 2910 (6 Mpa.s) /
Pharmaceutical Classes:
- Serotonin Reuptake Inhibitor [EPC]
- Serotonin Uptake Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 68788-4011 Fluvoxamine Maleate 100 mg Oral Tablet by Preferred Pharmaceuticals Inc.
- 68788-8175 Fluvoxamine Maleate 100 mg Oral Tablet by Preferred Pharmaceuticals Inc.
- 0185-0017 Fluvoxamine Maleate 25 mg Oral Tablet by Eon Labs, Inc.
- 0185-0027 Fluvoxamine Maleate 50 mg Oral Tablet by Eon Labs, Inc.
- 0185-0157 Fluvoxamine Maleate 100 mg Oral Tablet by Eon Labs, Inc.
- 0228-2848 Fluvoxamine Maleate 100 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0228-2849 Fluvoxamine Maleate 150 mg Oral Capsule, Extended Release by Actavis Pharma, Inc.
- 0378-0407 Fluvoxamine Maleate 25 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0412 Fluvoxamine Maleate 50 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0414 Fluvoxamine Maleate 100 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0555-0967 Fluvoxamine Maleate 25 mg Oral Tablet by Barr Laboratories Inc.
- 0555-0968 Fluvoxamine Maleate 50 mg Oral Tablet by Barr Laboratories Inc.
- 0555-0969 Fluvoxamine Maleate 100 mg Oral Tablet by Barr Laboratories Inc.
- 0615-7569 Fluvoxamine Maleate 100 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0832-1670 Fluvoxamine Maleate 25 mg Oral Tablet, Film Coated by Upsher-smith Laboratories, LLC
- 0832-1671 Fluvoxamine Maleate 50 mg Oral Tablet, Film Coated by Upsher-smith Laboratories, LLC
- 0832-1672 Fluvoxamine Maleate 100 mg Oral Tablet, Film Coated by Upsher-smith Laboratories, LLC
- 10370-175 Fluvoxamine Maleate 100 mg 24 Hr Extended Release Capsule by Par Pharmaceutical, Inc.
- 10370-176 Fluvoxamine Maleate 150 mg 24 Hr Extended Release Capsule by Par Pharmaceutical, Inc.
- 13668-290 Fluvoxamine Maleate 100 mg Oral Capsule, Extended Release by Torrent Pharmaceuticals Limited
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 68788-7218Next: 68788-7220 >
Related Discussions:
FLUVOXAMINE 100
I HAVE BEEN ON AN ANTIDEPRESAND FOR A YEAR NOW. SINCE I STARTED I HAVE HAD BODY PAINS. MY DOCTOR TOLD ME IT IS A MEDICAL...
I HAVE BEEN ON AN ANTIDEPRESAND FOR A YEAR NOW. SINCE I STARTED I HAVE HAD BODY PAINS. MY DOCTOR TOLD ME IT IS A MEDICAL...
fluvoxamine e27 50mg
round yellow tablet has e27 on one side...
round yellow tablet has e27 on one side...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.