68071-5080 : Pioglitazone 30 mg Oral Tablet
| NDC: | 68071-5080 |
| Labeler: | Nucare Pharmaceuticals, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Pioglitazone Pioglitazone |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA077210 |
| Rev. Date: |
Appearance:
| Markings: | TEVA;7272 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 7 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
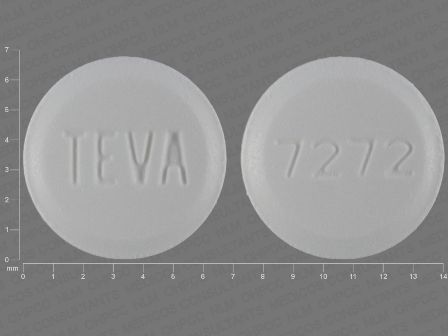
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 68071-5080-3: 30 TABLET IN 1 BOTTLE (68071‑5080‑3)
Active Ingredients:
- Pioglitazone Hydrochloride
Dosage Strength:
- 30 mg
Inactive Ingredients:
- Carboxymethylcellulose Calcium
- Hydroxypropyl Cellulose (1600000 Wamw)
- Magnesium Stearate
- Mannitol /
Pharmaceutical Classes:
- PPAR alpha [CS]
- PPAR gamma [CS]
- Peroxisome Proliferator Receptor alpha Agonist [EPC]
- Peroxisome Proliferator Receptor gamma Agonist [EPC]
- Peroxisome Proliferator-activated Receptor Activity [MoA]
- Thiazolidinedione [EPC]
- Thiazolidinediones [CS]
Related Products:
Based on records with the same trade name.- 68071-1621 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-2264 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-2373 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-2437 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-2495 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-2886 Pioglitazone 15 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-4959 Pioglitazone 15 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-4964 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-5155 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-5199 Pioglitazone 15 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-5210 Pioglitazone 30 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 68071-5245 Pioglitazone 15 mg Oral Tablet by Nucare Pharmaceuticals, Inc.
- 0093-2046 Pioglitazone (As Pioglitazone Hydrochloride) 45 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-2047 Pioglitazone (As Pioglitazone Hydrochloride) 30 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-2048 Pioglitazone (As Pioglitazone Hydrochloride) 15 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7271 Pioglitazone 15 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7272 Pioglitazone 30 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7273 Pioglitazone 45 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0378-0048 Pioglitazone (As Pioglitazone Hydrochloride) 15 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0228 Pioglitazone (As Pioglitazone Hydrochloride) 30 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 68071-5079Next: 68071-5081 >
Related Discussions:
Pioglitazone HCl-piozone
what are the side effects of piozone? ## usage and indications of piozone 15 mg ## Piozone contains the active ingredien... 2 replies
what are the side effects of piozone? ## usage and indications of piozone 15 mg ## Piozone contains the active ingredien... 2 replies
Has Pioglitazone been discontinued?
I was told by my drugstore, Walgreens, that Pioglitazone has been discontinued. I wanted to find out if all drug compani... 2 replies
I was told by my drugstore, Walgreens, that Pioglitazone has been discontinued. I wanted to find out if all drug compani... 2 replies
Taking Pioglitazone, Metformin and Glimipride Together
Is it safe to use pioglitazone, metformin and glimipride together for managing blood sugar levels? ## Yes, they can be u... 1 reply
Is it safe to use pioglitazone, metformin and glimipride together for managing blood sugar levels? ## Yes, they can be u... 1 reply
Voglibose 0.2 & pioglitazone 7.5
Hello, my doctor changed my medicine from metformin sr 500 mg to Voglibose 0.2 & pioglitazone 7.5 both at night. pre... 1 reply
Hello, my doctor changed my medicine from metformin sr 500 mg to Voglibose 0.2 & pioglitazone 7.5 both at night. pre... 1 reply
what are the side effects of pioglitazone hydrochloride plus metaphormin
I AM A SUGAR PATIENT AND DR PRESCRIBED ME PIOZMF-30 ABOUT 2 YEARS BACK. IMMEDIATELLY AFTER A MONTH OF USE I DEVELOPED SK... 1 reply
I AM A SUGAR PATIENT AND DR PRESCRIBED ME PIOZMF-30 ABOUT 2 YEARS BACK. IMMEDIATELLY AFTER A MONTH OF USE I DEVELOPED SK... 1 reply
Taking Nesina with Glimepiride, Pioglitazone, and Metformin
Is it safe to take Nesina with Glimepiride, Pioglitazone, and Metformin? ## As long as your doctor has prescribed them a... 1 reply
Is it safe to take Nesina with Glimepiride, Pioglitazone, and Metformin? ## As long as your doctor has prescribed them a... 1 reply
pioglitazone hci
zolid 30/500 mg ## Pioglitazone is an oral antidiabetic medications, used to help control blood sugar. Side effects can ... 1 reply
zolid 30/500 mg ## Pioglitazone is an oral antidiabetic medications, used to help control blood sugar. Side effects can ... 1 reply
Pioglitazone hcl and Metformin hcl 15/850md of Sandoz
Hi, good to see this section on this site. I am looking for this diabetes med. The bottle has 60 tablets in it. How can ...
Hi, good to see this section on this site. I am looking for this diabetes med. The bottle has 60 tablets in it. How can ...
Actos pioglitazone
white round tablet used as a water pill ## Actos This is NOT a water pill! This is a diabetes med to help control blood ... 2 replies
white round tablet used as a water pill ## Actos This is NOT a water pill! This is a diabetes med to help control blood ... 2 replies
Gilmepiride 2mg pioglitazone 7.5 mg and metformin HCL (SR) 1000 mg
My Father changed his medicine from glimepiride 2mg and metformine 500 mg to Gilmepiride 2mg pioglitazone 7.5 mg and met...
My Father changed his medicine from glimepiride 2mg and metformine 500 mg to Gilmepiride 2mg pioglitazone 7.5 mg and met...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.