68071-4254 : Aspirin 81 mg Enteric Coated 81 mg Enteric Coated 81 mg Oral Tablet
| NDC: | 68071-4254 |
| Labeler: | Nucare Pharmaceuticals, Inc. |
| Product Type: | Human OTC Drug |
| Drug Name: |  Aspirin 81 mg Enteric Coated Aspirin 81 mg Enteric Coated |
| Dosage Form: | Oral Tablet |
| Application #: | part343 |
| Rev. Date: |
Appearance:
| Markings: | AP;121 |
| Shapes: |
Round |
| Colors: |
 Yellow Yellow |
| Size (mm): | 8 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
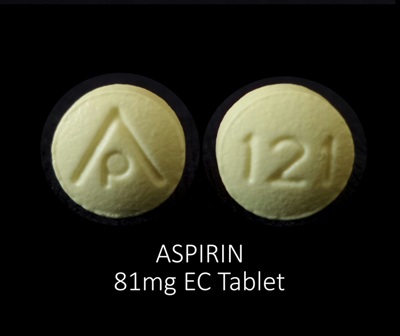
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 68071-4254-1: 100 TABLET IN 1 BOTTLE (68071‑4254‑1)
- 68071-4254-2: 120 TABLET IN 1 BOTTLE (68071‑4254‑2)
- 68071-4254-9: 90 TABLET IN 1 BOTTLE (68071‑4254‑9)
Active Ingredients:
- Aspirin
Dosage Strength:
- 81 mg
Inactive Ingredients:
- Cellulose, Microcrystalline
- Propylene Glycol
- Starch, Corn
- Stearic Acid
- Titanium Dioxide
- Methacrylic Acid
- D&c Yellow No. 10
- Polyethylene Glycol 1000
- Silicon Dioxide
- Talc
- Croscarmellose Sodium
- Hypromelloses
- Povidone
- Sodium Lauryl Sulfate /
Related Products:
Based on records with the same trade name.- 10135-173 Aspirin 81 mg Enteric Coated 81 mg Enteric Coated 81 mg Oral Tablet by Marlex Pharmaceuticals Inc
- 10135-689 Aspirin 81 mg Enteric Coated 81 mg Enteric Coated 81 mg Oral Tablet by Marlex Pharmaceuticals Inc
- 55700-746 Aspirin 81 mg Enteric Coated 81 mg Enteric Coated 81 mg Oral Tablet by Quality Care Products, LLC
- 69618-017 Aspirin 81 mg Enteric Coated 81 mg Enteric Coated 81 mg Oral Tablet by Reliable 1 Laboratories LLC
- 69618-046 Aspirin 81 mg Enteric Coated 81 mg Enteric Coated 81 mg Oral Tablet by Reliable 1 Laboratories LLC
- 70518-1649 Aspirin 81 mg Enteric Coated 81 mg Enteric Coated 81 mg Oral Tablet by Remedyrepack Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 68071-4253Next: 68071-4255 >
Related Discussions:
Aspirin (Aspilets) 80mg tablets
What is the use of yellow Aspilets 80mg tablets? ## This contains the active ingredient Aspirin, it is used as a pain re... 14 replies
What is the use of yellow Aspilets 80mg tablets? ## This contains the active ingredient Aspirin, it is used as a pain re... 14 replies
Aspirin-aspilets 80mg pregnancy safety
I'm 20 weeks pregnant now and my doctor gave me aspilets 80mg to prevent from being pre-eclamsia because I had a sev... 3 replies
I'm 20 weeks pregnant now and my doctor gave me aspilets 80mg to prevent from being pre-eclamsia because I had a sev... 3 replies
Aspirin Tablets 75 mg. Enteric Coated
75 mg Enteric Coated ## what are ide effects of 75mg enteric coated aspirin tablet.should it be taken on empty stomach o... 3 replies
75 mg Enteric Coated ## what are ide effects of 75mg enteric coated aspirin tablet.should it be taken on empty stomach o... 3 replies
aspirin gastro resistance tablet and delayed release tablet
Hi. I was taking aspirin delayed release 75mg. For last 3-4 years. As the tablet got over I requested one of my friend t... 2 replies
Hi. I was taking aspirin delayed release 75mg. For last 3-4 years. As the tablet got over I requested one of my friend t... 2 replies
aspirin 81 mg and pristiq 50 mg
Should I be taking the above medications daily? ## Can I take 50 mg pristiq, 2.5 mg indapamide and 81 mg aspirin daily #... 2 replies
Should I be taking the above medications daily? ## Can I take 50 mg pristiq, 2.5 mg indapamide and 81 mg aspirin daily #... 2 replies
Aspirin In Hydrocodone Acetaminophen 5 325
all I need to know is "does the above mentioned RX have aspirin in it. Thanks ## Hi Jessie, The medication containin... 2 replies
all I need to know is "does the above mentioned RX have aspirin in it. Thanks ## Hi Jessie, The medication containin... 2 replies
Aspirin Tablet 80mg
Dear Sir, Can Aspirin Tablet 80mg (2 tablet a day) be taken together with Metformin HC1 Tablet 500mg (0.5 tablet X 3 tim... 2 replies
Dear Sir, Can Aspirin Tablet 80mg (2 tablet a day) be taken together with Metformin HC1 Tablet 500mg (0.5 tablet X 3 tim... 2 replies
Aspirin 80 mg Tab (Aspilets 80mg)
I am 76 years old,I had a coronary - angiography with the overall conclusions as follows :Calcium Score 34.5 Coronary ar... 2 replies
I am 76 years old,I had a coronary - angiography with the overall conclusions as follows :Calcium Score 34.5 Coronary ar... 2 replies
aspirin/cal/mag
Why team up aspirin with cal/mag? ## Hi Theo, I'm not quite sure what you mean, are you wondering why this this medi... 2 replies
Why team up aspirin with cal/mag? ## Hi Theo, I'm not quite sure what you mean, are you wondering why this this medi... 2 replies
Aspirin + Xanax = Constipation?
Can 325mg of Aspirin & 0.25 mg of Xanax a day cause constipation? ## It could be one of the medications, a combinati... 2 replies
Can 325mg of Aspirin & 0.25 mg of Xanax a day cause constipation? ## It could be one of the medications, a combinati... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.