63629-5292 : Brilinta 90 mg Oral Tablet
| NDC: | 63629-5292 |
| Labeler: | Bryant Ranch Prepack |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Brilinta Brilinta |
| Dosage Form: | Oral Tablet |
| Application #: | NDA022433 |
| Rev. Date: |
Appearance:
| Markings: | 90;T |
| Shapes: |
Round |
| Colors: |
 Yellow Yellow |
| Size (mm): | 9 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
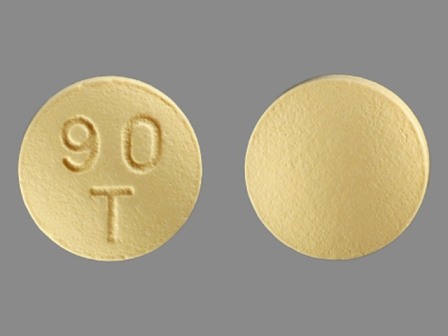
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 63629-5292-1: 30 TABLET IN 1 BOTTLE (63629‑5292‑1)
Active Ingredients:
- Ticagrelor
Dosage Strength:
- 90 mg
Inactive Ingredients:
- Mannitol
- Calcium Phosphate, Dibasic, Dihydrate
- Sodium Starch Glycolate Type a Potato
- Hydroxypropyl Cellulose
- Magnesium Stearate
- Water
- Hypromelloses
- Titanium Dioxide
- Talc
- Polyethylene Glycol 400
- Ferric Oxide Yellow
Pharmaceutical Classes:
- P2Y12 Platelet Inhibitor [EPC]
- Phenylalanine Hydroxylase Activators [MoA]
- Decreased Platelet Aggregation [PE]
- P2Y12 Receptor Antagonists [MoA]
- Cytochrome P450 3A4 Inhibitors [MoA]
- Cytochrome P450 3A5 Inhibitors [MoA]
- P-Glycoprotein Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 0186-0776 Brilinta 90 mg Oral Tablet by Astrazeneca Lp
- 0186-0777 Brilinta 90 mg Oral Tablet by Astrazeneca Lp
- 55154-9618 Brilinta 90 mg Oral Tablet by Cardinal Health
- 71610-815 Brilinta 90 mg Oral Tablet by Aphena Pharma Solutions - Tennessee, LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 63629-5291Next: 63629-5302 >
Related Discussions:
Ticagrelor, Breathlessness
My father suffered from a heart attack 3 weeks ago and now they put him on Ticagrelor 90mg twice daily. Up until having ... 2 replies
My father suffered from a heart attack 3 weeks ago and now they put him on Ticagrelor 90mg twice daily. Up until having ... 2 replies
Ticagrelor and OxyContin
Is it ok to take Ticagrelor (Brilinta) 90 mg and OxyContin together? ## According to the NIH I am not seeing any interac... 1 reply
Is it ok to take Ticagrelor (Brilinta) 90 mg and OxyContin together? ## According to the NIH I am not seeing any interac... 1 reply
BRILINTA CAUSES SHORTAGE OF BREATH?
HELLO. MY DAD GOT A STENT LAST MONTH. THEREFORE NOW HE HAS TO TAKE BRLINTA...but now he doesn't have energy..gets di... 5 replies
HELLO. MY DAD GOT A STENT LAST MONTH. THEREFORE NOW HE HAS TO TAKE BRLINTA...but now he doesn't have energy..gets di... 5 replies
Brilinta issues
Hi I have had 2 stents placed around 2 weeks back and was put on Brilinita 2 tablets of 90 mg each per day. Ever since I... 2 replies
Hi I have had 2 stents placed around 2 weeks back and was put on Brilinita 2 tablets of 90 mg each per day. Ever since I... 2 replies
Brilinta now causing oxygen therapy 24/7
My father had a stent placed in dec 2017. Immediately following procedure he said he couldn’t breathe. They put hi... 2 replies
My father had a stent placed in dec 2017. Immediately following procedure he said he couldn’t breathe. They put hi... 2 replies
Brilinta with Lipitor
I had a heart attack 5 years ago with two drug eluted stents installed I was on plavix for 3 years with no problem .my c... 2 replies
I had a heart attack 5 years ago with two drug eluted stents installed I was on plavix for 3 years with no problem .my c... 2 replies
Brilinta and muscle discomfort
I have been on Brilinta for 2 days at 90 mg , 2x per day and i have this muscle discomfort below my right armpit and som... 1 reply
I have been on Brilinta for 2 days at 90 mg , 2x per day and i have this muscle discomfort below my right armpit and som... 1 reply
Brilinta vs Plavix
Brilinta is newer than Plavix and a superior medicine if you can tolerate it. I experienced dizziness, confusion, and ch...
Brilinta is newer than Plavix and a superior medicine if you can tolerate it. I experienced dizziness, confusion, and ch...
Alcohol and Brilinta
Can you consume alcohol while taking Brilinta? What are your experiences? Thank you ## It would be best not to imbibe, w... 1 reply
Can you consume alcohol while taking Brilinta? What are your experiences? Thank you ## It would be best not to imbibe, w... 1 reply
High Altitudes & Brilinta
If you take Brilinta twice a day like 90 or 60 mg tablets, keep out of the higher elevations like 6500' and up. Had ... 2 replies
If you take Brilinta twice a day like 90 or 60 mg tablets, keep out of the higher elevations like 6500' and up. Had ... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.