59762-4440 : Methylprednisolone 4 mg Oral Tablet 21 Count Pack
| NDC: | 59762-4440 |
| Labeler: | Greenstone LLC |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Methylprednisolone Methylprednisolone |
| Dosage Form: | Oral Tablet |
| Application #: | NDA011153 |
| Rev. Date: |
Appearance:
| Markings: | MEDROL;4 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 8 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
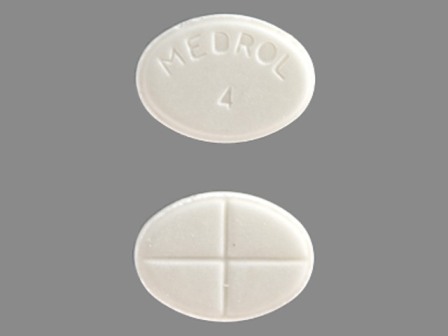
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 59762-4440-2: 1 DOSE PACK IN 1 CARTON (59762‑4440‑2) > 21 TABLET IN 1 DOSE PACK
- 59762-4440-3: 100 TABLET IN 1 BOTTLE (59762‑4440‑3)
Active Ingredients:
- Methylprednisolone
Dosage Strength:
- 4 mg
Inactive Ingredients:
- Calcium Stearate
- Starch, Corn
- Lactose
- Sucrose
Pharmaceutical Classes:
- Corticosteroid Hormone Receptor Agonists [MoA]
- Corticosteroid [EPC]
Related Products:
Based on records with the same trade name.- 59762-0049 Methylprednisolone 8 mg Oral Tablet by Greenstone LLC
- 59762-0050 Methylprednisolone 16 mg Oral Tablet by Greenstone LLC
- 59762-0051 Methylprednisolone 32 mg Oral Tablet by Greenstone LLC
- 59762-3327 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Greenstone LLC
- 0179-0124 Methylprednisolone 16 mg Oral Tablet by Kaiser Foundation Hospitals
- 0591-0790 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Watson Labs
- 0603-4593 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Qualitest Pharmaceuticals
- 0703-0043 Methylprednisolone 40 mg/ml Intramuscular; Intrasynovial; Soft Tissue Injection by Teva Parenteral Medicines, Inc
- 0703-0045 Methylprednisolone 40 mg/ml Intramuscular Injection by Teva Parenteral Medicines, Inc
- 0703-0063 Methylprednisolone 80 mg/ml Intramuscular; Intrasynovial; Soft Tissue Injection by Teva Parenteral Medicines, Inc
- 0781-5022 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Sandoz Inc
- 0904-6574 Methylprednisolone 4 mg Oral Tablet by Major Pharmaceuticals
- 0904-6914 Methylprednisolone 4 mg Oral Tablet by Major Pharmaceuticals
- 16590-149 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Stat Rx USA LLC
- 21695-080 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Rebel Distributors Corp.
- 33261-335 Methylprednisolone 4 mg/1 Oral Tablet by Aidarex Pharmaceuticals LLC
- 35356-763 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Lake Erie Medical Dba Quality Care Products LLC
- 42549-522 Methylprednisolone 4 mg Oral Tablet 21 Count Pack by Stat Rx USA LLC
- 42806-400 Methylprednisolone 4 mg Oral Tablet by Epic Pharma, LLC
- 45865-104 Methylprednisolone 4 mg Oral Tablet by Medsource Pharmaceuticals
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 59762-4355Next: 59762-4460 >
Related Discussions:
Methylprednisolone Tablets Usp 4 Mg side effects
What are the possible side effects of Methylprednisolone 4mg tablets? ## Methylprednisolone is a corticosteroid drug tha... 40 replies
What are the possible side effects of Methylprednisolone 4mg tablets? ## Methylprednisolone is a corticosteroid drug tha... 40 replies
Methylprednisolone 4mg pak for sinus infection along with cephalexin 500mg (twice daily)
I took Methylpredinisolone 4mg pak for 6 days, like 2 days later my headaches are so bad and its all day, yesterday head... 7 replies
I took Methylpredinisolone 4mg pak for 6 days, like 2 days later my headaches are so bad and its all day, yesterday head... 7 replies
Methylprednisolone Nausea a week later?
Last week I stopped the 6 day course on day three because of sever side effects. I took this medicine two months ago I f... 6 replies
Last week I stopped the 6 day course on day three because of sever side effects. I took this medicine two months ago I f... 6 replies
methylprednisolone 4mg dose pack
I was given the 4mg 5 day dose pack of methylprednisolone for an allergic reaction that caused swelling of my lips and f... 4 replies
I was given the 4mg 5 day dose pack of methylprednisolone for an allergic reaction that caused swelling of my lips and f... 4 replies
Methylprednisolone 500mg
my sons MRI scan has shown inflamation/swelling of the lining of his spinal cord near his neck. he is seeing a neurologi... 4 replies
my sons MRI scan has shown inflamation/swelling of the lining of his spinal cord near his neck. he is seeing a neurologi... 4 replies
methylprednisolone 4mg tab
I am curious, why not methylprednisolone tab 2 or 4mg is not available in Dublin..since it is used in patients with infl... 4 replies
I am curious, why not methylprednisolone tab 2 or 4mg is not available in Dublin..since it is used in patients with infl... 4 replies
methylprednisolone talet 4 m.g.
## I was just prescribed this med. and am afraid to take it. ## Why are you afraid to take it? It is generic prednisone ... 4 replies
## I was just prescribed this med. and am afraid to take it. ## Why are you afraid to take it? It is generic prednisone ... 4 replies
Methylprednisolone tablet 4 mg
Hi I'm deb, I have been prescribed methylprednisone 4mg for plantar fasciitis in my foot.. take 5 then 4,3,2,then 1 ... 3 replies
Hi I'm deb, I have been prescribed methylprednisone 4mg for plantar fasciitis in my foot.. take 5 then 4,3,2,then 1 ... 3 replies
Methylprednisolone pack + Flonase = No more allergies or anxiety!
I have had nothing but positive experiences since I started taking methylprednisolone for my allergies. I went to the do... 3 replies
I have had nothing but positive experiences since I started taking methylprednisolone for my allergies. I went to the do... 3 replies
Methylprednisolone dosage pack
I was prescribed methylprednisolone yesterday and took day all 6 pills, now I have a horrible headache. I've had ulc... 2 replies
I was prescribed methylprednisolone yesterday and took day all 6 pills, now I have a horrible headache. I've had ulc... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.