55700-412 : Tramadol Hydrochloride 50 mg Oral Tablet
| NDC: | 55700-412 |
| Labeler: | Lake Erie Medical Dba Quality Care Products LLC |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Tramadol Hydrochloride Tramadol Hydrochloride |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA091498 |
| Rev. Date: | |
| CSA Schedule: | CIV (US) [1] |
[1] Schedule IV Controlled Substance: Low potential for abuse relative to substances in Schedule III. Examples include Alprazolam (Xanax), Diazepam (Valium), Carisoprodol (Soma), Clonazepam (Klonopin), Lorazepam (Ativan), Clorazepate (Tranxene), Midazolam (Versed), Temazepam (Restoril), and Triazolam (Halcion).. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | 101;OUYI |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 10 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
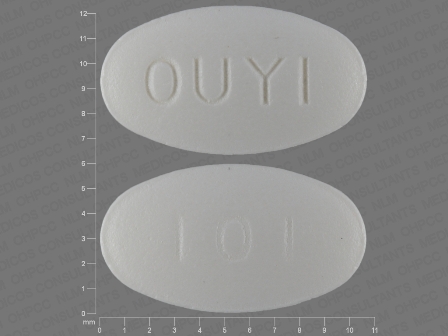
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55700-412-01: 120 TABLET IN 1 BOTTLE (55700‑412‑01)
- 55700-412-08: 8 TABLET IN 1 BOTTLE (55700‑412‑08)
- 55700-412-12: 12 TABLET IN 1 BOTTLE (55700‑412‑12)
- 55700-412-15: 15 TABLET IN 1 BOTTLE (55700‑412‑15)
- 55700-412-18: 180 TABLET IN 1 BOTTLE (55700‑412‑18)
- 55700-412-20: 20 TABLET IN 1 BOTTLE (55700‑412‑20)
- 55700-412-24: 240 TABLET IN 1 BOTTLE (55700‑412‑24)
- 55700-412-30: 30 TABLET IN 1 BOTTLE (55700‑412‑30)
- 55700-412-40: 40 TABLET IN 1 BOTTLE (55700‑412‑40)
- 55700-412-60: 60 TABLET IN 1 BOTTLE (55700‑412‑60)
- 55700-412-90: 90 TABLET IN 1 BOTTLE (55700‑412‑90)
Active Ingredients:
- Tramadol Hydrochloride
Dosage Strength:
- 50 mg
Inactive Ingredients:
- Starch, Corn
- Hypromelloses
- Lactose Monohydrate
- Magnesium Stearate
- Cellulose, Microcrystalline
- Polyethylene Glycols
- Polyvinyl Alcohol
- Talc
- Titanium Dioxide
Pharmaceutical Classes:
- Full Opioid Agonists [MoA]
- Opioid Agonist [EPC]
Related Products:
Based on records with the same trade name.- 55700-016 Tramadol Hydrochloride 200 mg 24 Hr Extended Release Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 55700-029 Tramadol Hydrochloride 100 mg Oral Tablet, Extended Release by Lake Erie Medical Dba Quality Care Products LLC
- 55700-293 Tramadol Hydrochloride 150 mg Oral Capsule, Extended Release by Lake Erie Medical Dba Quality Care Products LLC
- 55700-864 Tramadol Hydrochloride 50 mg Oral Tablet by Quality Care Products, LLC
- 55700-996 Tramadol Hydrochloride 100 mg Oral Tablet, Extended Release by Quality Care Products, LLC
- 55700-997 Tramadol Hydrochloride 50 mg Oral Tablet, Coated by Quality Care Products, LLC
- 35356-546 Tramadol Hydrochloride 100 mg 24 Hr Extended Release Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 35356-547 Tramadol Hydrochloride 200 mg 24 Hr Extended Release Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 35356-659 Tramadol Hydrochloride 50 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 35356-767 Tramadol Hydrochloride 150 mg 24 Hr Extended Release Capsule by Lake Erie Medical Dba Quality Care Products LLC
- 35356-790 Tramadol Hydrochloride 300 mg 24 Hr Extended Release Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 49999-129 Tramadol Hydrochloride 50 mg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 0093-0058 Tramadol Hydrochloride 50 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-3301 Tramadol Hydrochloride 50 mg Oral Tablet, Film Coated by Teva Pharmaceuticals USA, Inc.
- 0228-2714 Tramadol Hydrochloride 50 mg Oral Tablet by Actavis
- 0378-4151 Tramadol Hydrochloride 50 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-4152 Tramadol Hydrochloride 100 mg Oral Tablet, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-4153 Tramadol Hydrochloride 200 mg Oral Tablet, Extended Release by Mylan Pharmaceuticals Inc.
- 0378-4154 Tramadol Hydrochloride 300 mg Oral Tablet, Extended Release by Mylan Pharmaceuticals Inc.
- 0615-7810 Tramadol Hydrochloride 50 mg Oral Tablet, Film Coated by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55700-411Next: 55700-413 >
Related Discussions:
Tramadol HCL 50 MG TAB MYLAN
one side of pill is a M and the other side of the pill is T7 ## what type of drug is tramadol hcl 50 mg. is it narcotic,... 152 replies
one side of pill is a M and the other side of the pill is T7 ## what type of drug is tramadol hcl 50 mg. is it narcotic,... 152 replies
Tramadol by Amneal vs Mylan
All our pharmacies here have switched to amneal, which doesn't do a thing except give a headache. The brand does mak... 109 replies
All our pharmacies here have switched to amneal, which doesn't do a thing except give a headache. The brand does mak... 109 replies
tramadol after August 18??
Hearing lots of places that the people who order tramadol via internet will no longer be able to. I live in a town with ... 99 replies
Hearing lots of places that the people who order tramadol via internet will no longer be able to. I live in a town with ... 99 replies
Tramadol 50 mg from Zenlabs
Hello all. I usually take Vicodin when I have bad back pain, but wanted to try something that wouldn't knock me out.... 86 replies
Hello all. I usually take Vicodin when I have bad back pain, but wanted to try something that wouldn't knock me out.... 86 replies
Tramadol classification
Is Tramadol a controlled substance, or is there any type of narcotic in them? I would really appreciate any reply. I wou... 77 replies
Is Tramadol a controlled substance, or is there any type of narcotic in them? I would really appreciate any reply. I wou... 77 replies
Tramadol Controlled Substance?
Is Tramadol considered to be a controlled medication? A narc med? ## I truly believe Tramadol is an addictive medication... 45 replies
Is Tramadol considered to be a controlled medication? A narc med? ## I truly believe Tramadol is an addictive medication... 45 replies
Tramadol withdrawal terrible stomach pain
Hello. I have searched the web and can't seem to find a problem like mine while coming off tramadol. I have horrible... 39 replies
Hello. I have searched the web and can't seem to find a problem like mine while coming off tramadol. I have horrible... 39 replies
Tramadol withdrawal side effects
My husband takes Tramadol for pain. It has caused him to be depressed. He is now addicted to the drug can't function... 31 replies
My husband takes Tramadol for pain. It has caused him to be depressed. He is now addicted to the drug can't function... 31 replies
tramadol hab pharma
I take ol-tram 100mg for chronic pain issues. From HAB pharmaceuticals. I open the capsule and tip it in my mouth, becau... 27 replies
I take ol-tram 100mg for chronic pain issues. From HAB pharmaceuticals. I open the capsule and tip it in my mouth, becau... 27 replies
Tramadol addiction
TRAMADOL=DECEPTIVE POISON. this drug is one of the nastier little secrets of the painkiller industry. from my experience... 25 replies
TRAMADOL=DECEPTIVE POISON. this drug is one of the nastier little secrets of the painkiller industry. from my experience... 25 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.